Small lysosomes, Big problems, Great solutions: Lysosomes in control of metabolic diseases
Division 2: Liver and Digestive Health
Department of Genetics and Cell Biology,
Laboratory Medicine and Medical University Vienna, Austria
Background
Obesity, accompanied by the characteristics of metabolic syndrome, constitutes the greatest threat to global health, affecting 20-25% of the adult population. These patients are at risk to develop cardiovascular diseases, type 2 diabetes (T2D) and a spectrum of liver diseases ranging from simple steatosis to non-alcoholic steatohepatitis (NASH). Additionally, obese subjects have increased risk for other complications such as hypertension, dyslipidemia, osteoarthritis, and some cancers.
Chronic low-grade inflammation is thought to be the primary cause for the complications. However, while it is clear that the inflammatory response is induced by oxidative stress, the molecular and cellular mechanisms that trigger inflammation in some but not all obese individuals remain unclear. One piece of the puzzle is related to lysosomes and lysosomal enzymes. In the last decade, lysosomes have emerged as a nutrient signalling hub - both sensing and directing metabolic responses.
Not surprisingly, lysosomal dysfunction is detrimental, and associated with development of lysosomal storage diseases, neurodegenerative disorders, cancers and a wide range of metabolic diseases. Our scientific ambition is to unravel the function and regulation of lysosomes in metabolic diseases in order to assess patients at risk for developing complications and to develop novel translational approaches for treatment and prevention.
Major breakthroughs
Mechanistically, we have demonstrated that hypercholesteremia leads to increased levels of oxLDL, which accumulates in lysosomes and triggers the development of inflammation. The accumulation of oxLDL in lysosomes initiates lysosomal dysfunction and leads to increased exocytosis of lysosomal enzymes.
Based on these studies, we have demonstrated the predictive potential of antibodies against oxLDL for the development of NASH in humans and of the lysosomal enzyme Cathepsin D (CTSD) as an extremely powerful and the earliest non-invasive biomarker for NASH.
We have further translated these mechanistic studies to the development of dietary, pharmacological and immunological treatments for combating low-grade inflammation. We showed that targeting dietary cholesterol and immunization against oxLDL are exceptionally efficient tools to prevent NASH. We have also applied these treatments within the context of a rare neurological disease (NPC1) and within cancer. Additional to the direct effect of oxLDL on the lysosomes, we have demonstrated that it leads to increased secretion of CTSD (among other lysosomal enzymes) into plasma of mice and humans with metabolic diseases including NASH, T2D and cancer.
We further demonstrated that increased CTSD in the plasma is a trigger for systemic disturbances in lipid metabolism, altered immune function and disease progression. These latest data suggest that plasma lysosomal enzymes also regulate systemic metabolic and inflammatory pathways independent from their intracellular role. To inhibit CTSD in a non-toxic manner we initiated the development of a novel inhibitor, which specifically targets the extracellular fraction of CTSD (and not the intracellular fraction) (PCT/IB2018/059764). By using this unique and non-toxic compound, we demonstrated decreased steatosis and systemic inflammation as well as reduced plasma insulin levels in rats and hyperlipidemic mice.
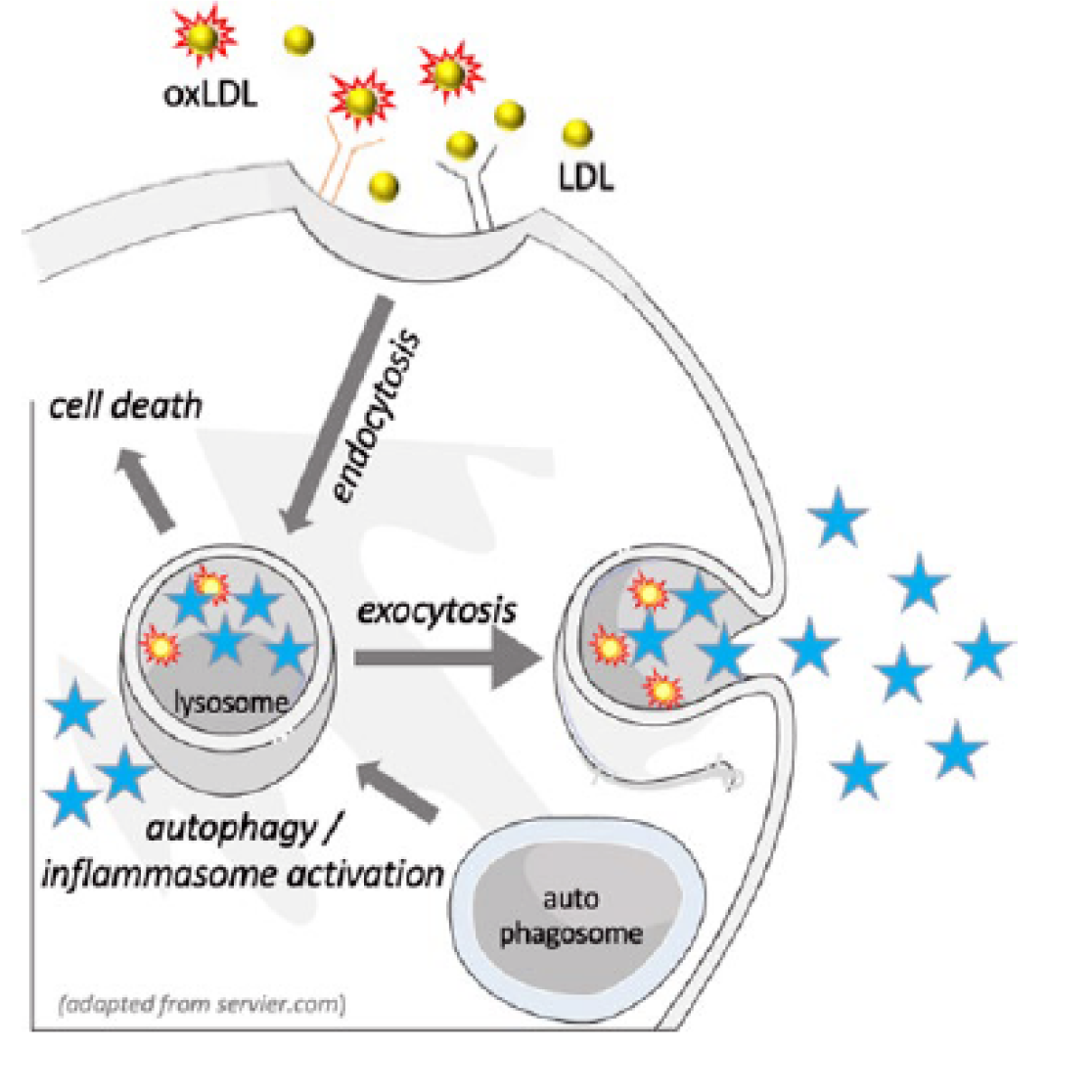
Figure 1: Rationale
Unlike non oxidized LDL, accumulation of oxLDL in lysosomes can lead to lysosomal dysfunction by interfering with autophagy, by triggering apoptosis or by increasing the secretion of lysosomal enzymes into the plasma.
Scientific impact/Research quality
Our data led to nine registered patent applications, which received wide interest from industrial partners and led to two clinical trials (1: dietary stanols as a treatment for NASH; 2: antibodies against oxLDL as a treatment for NPC1). Furthermore, it has been presented in multiple international conferences (i.e. ATLAS International Symposium, Denmark; Global NASH Congress, London; Complications of Diabetes and Obesity Symposium, Dublin, European Association for the Study of the Liver, Vienna; Leading-Edge Research Center for Drug Discovery, Kyungpook, South Korea) and was published in various high impact scientific journals.
Who is involved?
Multiple previous PhD students and postdocs contributed to the development of this research line. Our current team includes Prof. Dr. Ronit Shiri-Sverdlov (Principal investigator), Dr. Tom Houben (Assistant professor), Dr. Tim Hendrikx (Principal investigator), Dr. Albert Bitorina (Research assistant), Dr. Dennis Meesters (Lab manager and technician) and five PhD students; Tulasi Yadati, Lingling Ding, Ines Reis, Annemarie Westheim and Mengying Li.
The research was supported by multiple grants including NWO VENI, VIDI, TKI-LSH, CVON, VCK, KWF, CTMM, MLDS, Horizon 2020 and Novo-Nordisk Foundation.
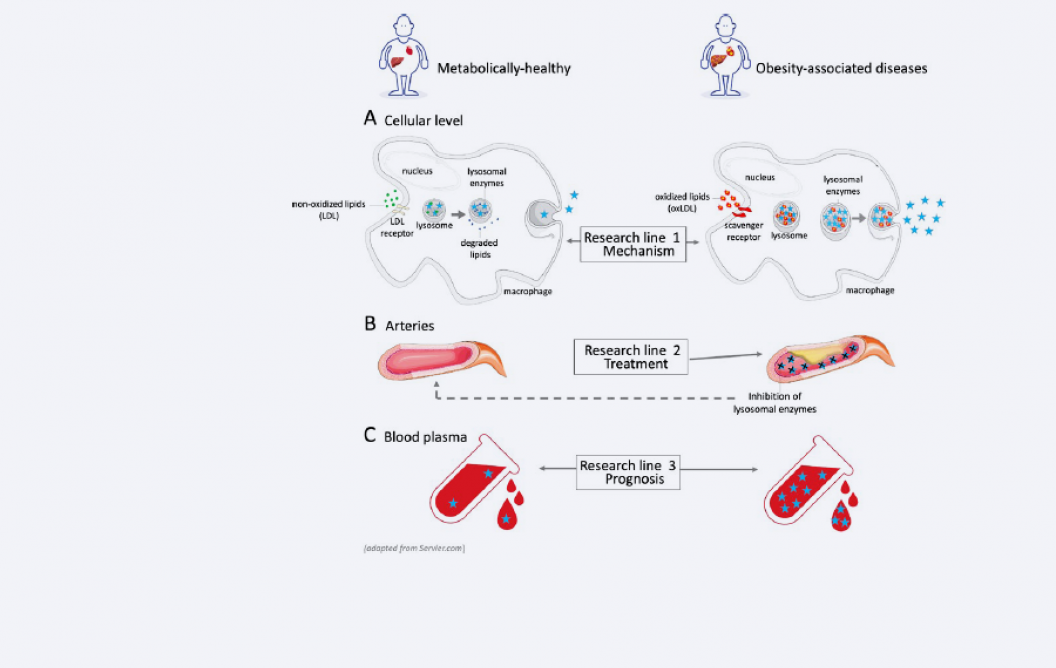
Figure 2: Research focus
Our research consists of three different lines:
- Mechanism
Investigation of the pathogenic signalling linking lysosomal dysfunction, disturbed lipid metabolism and inflammation. - Treatment
Developing novel treatments for obesity-associated diseases, aimed at improving lysosomal function and reducing the activity of circulating lysosomal enzymes. - Prognosis
Developing prognostic tools for assessing the risk of developing obesity-associated diseases.
Selection of recent publications
- Houben T, Oligschlaeger Y, Hendrikx T, Bitorina AV, Walenbergh SMA, van Gorp PJ, Gijbels MJJ, Friedrichs S, Plat J, Schaap FG, Lütjohann D, Hofker MH, Shiri-Sverdlov R; Cathepsin D regulates lipid metabolism in murine steatohepatitis. Sci Rep 2017 Jun;7(1):3494.
- Houben T, Magro Dos Reis I, Oligschlaeger Y, Steinbusch H, Gijbels MJJ, Hendrikx T, Binder CJ, Cassiman D, Westerterp M, Prickaerts J, Shiri-Sverdlov R; Pneumococcal immunization reduces neurological and hepatic symptoms in a mouse model for Niemann-Pick Type C1 disease. Front Immunol 2019 Jan;9:3089.
- Khurana P, Yadati T, Goyal S, Dolas A, Houben T, Oligschlaeger Y, Agarwal AK, Kulkarni A, Shiri-Sverdlov R; Inhibiting extracellular cathepsin D reduces hepatic steatosis in Sprague-Dawley rats. Biomolecules 2019 May;9(5):171.
- Ding L, Goossens GH, Oligschlaeger Y, Houben T, Blaak EE, Shiri-Sverdlov R; Plasma cathepsin D activity is negatively associated with hepatic insulin sensitivity in overweight and obese humans. Diabetologia 2020 Feb;63(2):374-84.
- Tom Houben, Albert V Bitorina, Yvonne Oligschlaeger, Mike LJ Jeurissen, Sander Rensen, Eleonore Köhler, Marit Westerterp, Dieter Lütjohann, Jan Theys, Andrea Romano, Jogchum Plat, Ronit Shiri-Sverdlov; Sex-opposed inflammatory effects of 27-hydroxycholesterol are mediated via differences in estrogen signaling. Journal of Pathology 2020 Aug;251(4):429-439.
Societal impact
Our work has been distributed via Layman communication platforms (i.e. Pan European Networks, LEVER, HashtageScishare, Atlas of Science and researchista.com) and involvements with the relevant national societies (i.e. NVH and MLDS).
Future Perspectives
Our aim is to explore the potential of targeting oxLDL and extracellular lysosomal enzymes as prognostic markers for disease severity and as non-toxic targets for treatment and for boosting the immune system in a wide variety of metabolic diseases including hepatocellular carcinoma, cardiovascular diseases, depression, Inflammatory bowel disease and autoimmune diseases.