Research Quality
Research Quality
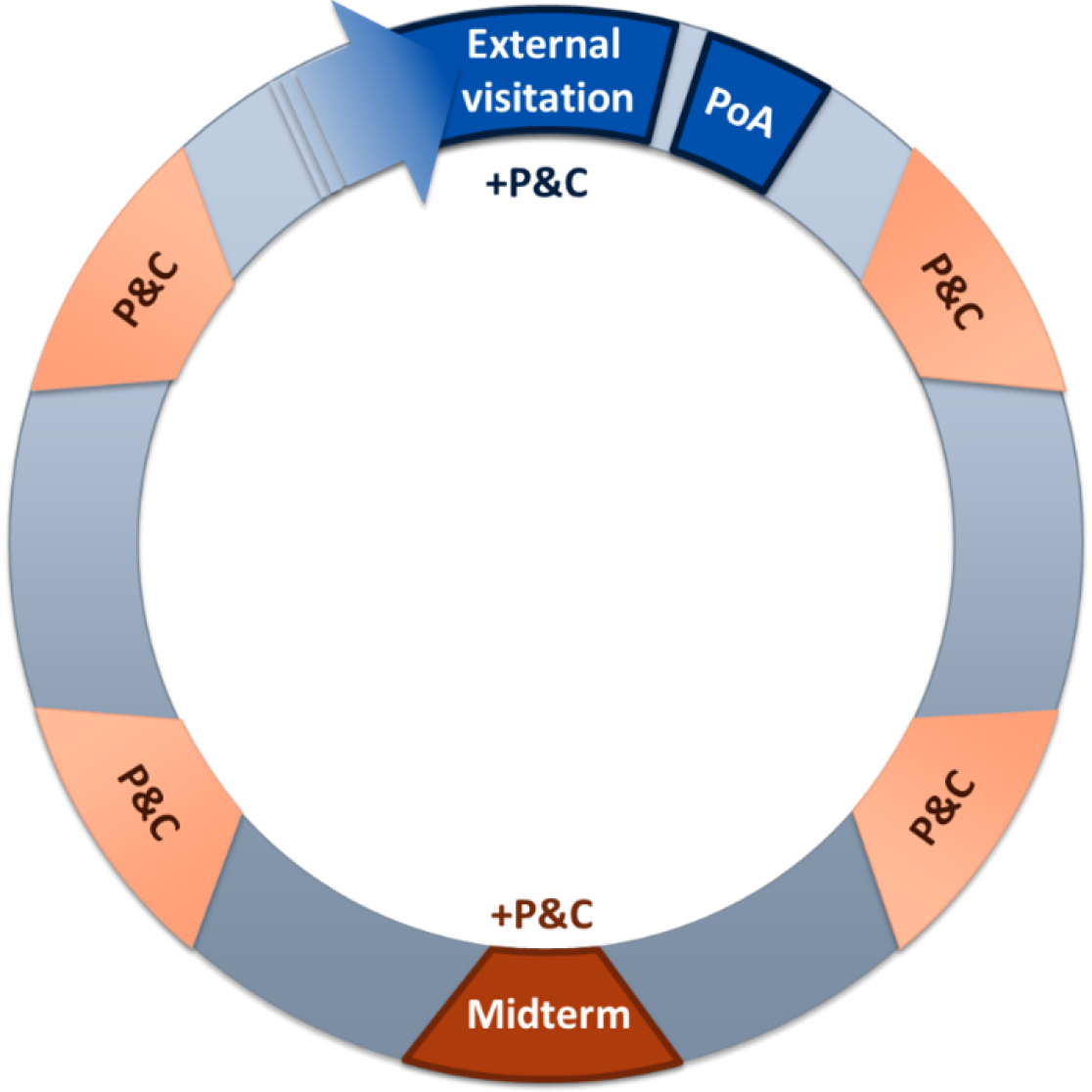
The quality of scientific research at the Faculty of Health, Medicine and Life Sciences (FHML) is evaluated by external, independent experts every six years. In Dutch universities, research evaluations follow the national Strategy Evaluation Protocol (SEP). At FHML the evaluations are conducted at the Research Institute level. To ensure an efficient workflow, FHML has established a structured quality assurance cycle for effective research evaluation. The cycle consists out of two main parts: the SEP and the Planning & Control (P&C).
Research Quality according to the SEP protocol
In accordance with the Strategy Evaluation Protocol (SEP) 2021-2027 concerning the quality of scientific research in the Netherlands, the university is obliged to compile a self-evaluation report over six year periods. The SEP aims to evaluate a Research Institute in light of its own aims and strategy. Hereby research quality, societal relevance, and viability are evaluated to provide feedback for improvement of the strategy and performance of the Research Unit.
Moreover, the Research Institute has to be evaluated by an external evaluation committee, which involves an External visitation in the form of a site visit. Following the site visit, the ERC will share an evaluation report including recommendations, based on which the Research Institute will create a Plan of Action (PoA).
Halfway through the six year period a Midterm review is conducted to sharpen objectives and adjust policies when required. The Midterm review is a compact version of the self-evaluation report, covering the first three years. It focuses on the response to and follow-up of the previous evaluation committee’s recommendations, as well as any changes in mission, ambitions, strategic goals compared to the previous evaluation and the strategy for the coming three years.
After the completion of the SEP evaluation, a summary of the unit’s self-evaluation report – including the case studies, the committee’s evaluation report, and the position document of the Board will be made publicly available as part of the quality assurance cycle. Please find these documents per Research Institute below:

CAPHRI - Care and Public Health Research Institute
CARIM - Cardiovascular Research Institute Maastricht
GROW - Research Institute for Oncology and Reproduction
Committee’s evaluation report (2018-2023) (will follow)
Position document (2018-2023) (will follow)
MERLN - Institute for Technology-Inspired Regenerative Medicine
MHeNs - Mental Health and Neuroscience Research Institute
NUTRIM - Institute for Nutrition and Translational Research in Metabolism
SHE - School of Health Professions Education
Committee’s evaluation report (2018-2023) (will follow)
Position document (2018-2023) (will follow)
Internal Planning & Control procedures
The FHML Planning & Control (P&C) procedures are a key component of the internal quality assurance cycle. Once a year, all FHML Research Institutes have P&C sessions with the FHML Board.
A key aspect of these meetings is aligning on strategic choices and potential policy changes for the coming years, ensuring continuous improvement and effective monitoring.
This continuous process helps Research Institutes remain actively engaged in quality assurance, making it a natural and structured part of their work. By collecting and reviewing key information annually, the institutes maintain an up-to-date overview of their progress, which also serves as preparation for the External visitation.