Pulmonary epithelial cells as central players in chronic lung disorders
Division 3: Respiratory & Age-related Health
Department of Respiratory Medicine
Department of Pharmacology and Toxicology
Department of Medical Microbiology
Background
According to estimates from the WHO and the Global Burden of Disease study, a staggering number of 500-600 million people worldwide suffer from chronic lung/airway diseases such as Chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), asthma and bronchopulmonary dysplasia (BPD). Chronic and repetitive exposure of the lungs and airways to insults such as noxious particles, allergens and infectious agents is considered the most important risk factor for developing these diseases.
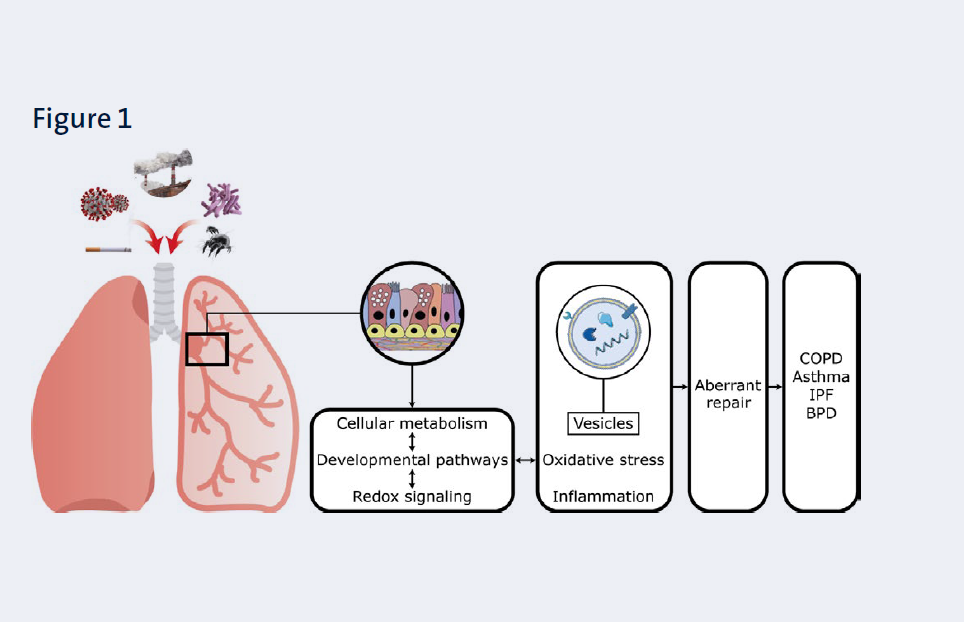
Our overall aim is to obtain insight in the underlying causes of maladaptive responses of respiratory epithelial cells to these insults from a molecular, cellular and whole tissue perspective with the ultimate goal of developing novel therapeutic strategies to reverse or halt disease progression. We focus on the following triggers: (components of) cigarette smoke, emissions from novel tobacco products (i.e. heated tobacco products), microplastics and wood smoke, allergens and infectious agents (Figure 1).
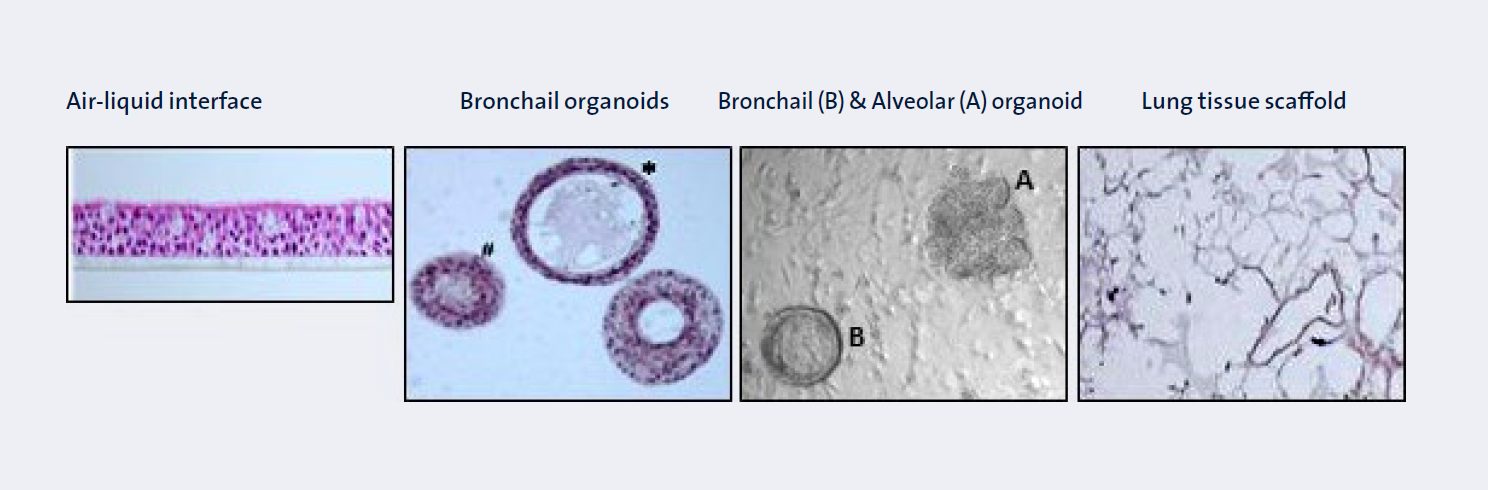
Our experimental models include state-of-the art human in vitro and ex vivo models as depicted in Figure 2, as well as primary human and murine stem cells, and precision cut lung slices.
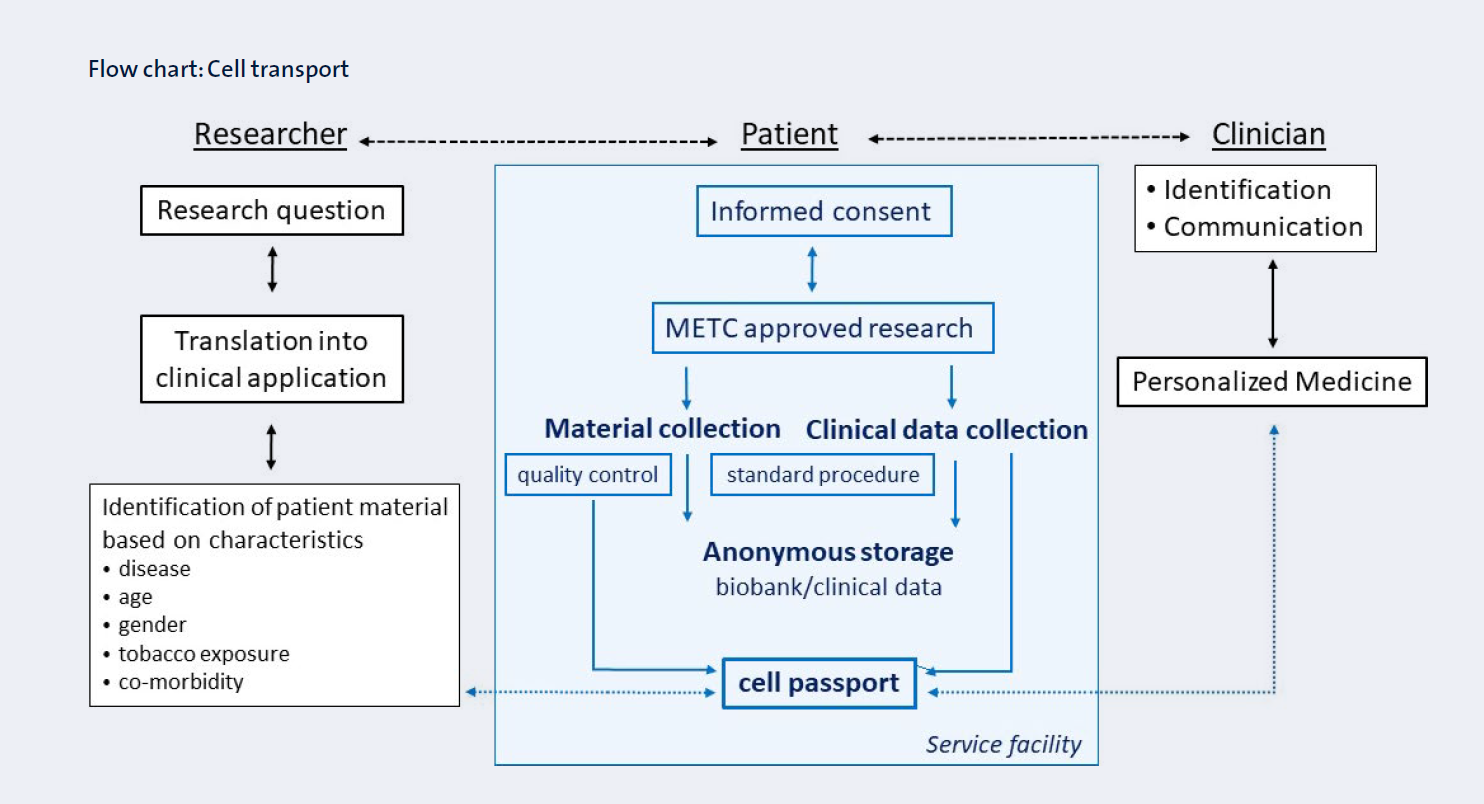
The Primary Lung Culture facility (PLUC) developed at the MUMC+ is essential for deployment of these models as it provides a unique biobank of well-characterized primary bronchial stem cells, alveolar stem cells, peripheral human lung tissues and primary fibroblasts (Figure 3).
Clinical evidence is obtained from these materials and, in addition, systemic biomarkers that can be used to monitor pulmonary processes are examined in clinical samples (mostly from the circulation and urine) which are available via our collaboration with the pulmonary rehabilitation center CIRO (Horn, the Netherlands). In addition, we use an extensive array of experimental animal models of disease and exposures mainly through our large network of collaborators (see below). An important read-out in our models is cellular metabolism (including glycolysis and mitochondrial function) as it is a key regulator of critical cellular processes and responses. We furthermore focus on the mediating role of redox signalling, the extracellular matrix (ECM), and intercellular communication via secreted factors such as extracellular vesicles (EVs)(Figure 1). Importantly, new therapeutic strategies, including pharmacological or cell-based therapies are tested.
Major breakthroughs
Cellular metabolism
Using peripheral lung tissue as well as primary human bronchial epithelial cells (PBECs) isolated from COPD and non-COPD donors, we have identified abnormalities in mitochondrial biogenesis and mitophagy in COPD likely induced by exposure of these cells to cigarette smoke (components). Furthermore, as a step towards future regulation of specific chemicals in cigarette smoke by governmental bodies, we have identified aldehydes, a harmful class of chemicals formed during the combustion and pyrolysis of tobacco, as compounds responsible for smoke-induced mitochondrial dysfunction in these cells. We are also working on extending these studies to include new, currently non-regulated, tobacco products such as heated tobacco products and are directing research efforts to explore the potential negative impact of inhaled microplastics and wood smoke (from woodstoves, a large contributor to airborne particulate matter in the Netherlands). In addition to these findings, we have identified abnormalities in the glycolysis pathway in animal models and clinical samples of asthmatics, and have demonstrated its crucial role in the pathogenesis of allergic airways disease by increasing IL-1β-induced proinflammatory signaling.
Redox signaling
We have shown the importance of redox regulation in general, and of the redox-based post-translational modification S-glutathionylation in particular, in modulating inflammation, cellular metabolism, and innate epithelial responses in relation to the diseases mentioned above. Importantly, we focus on modifiable redox events, offering the possibility to be (personalized) therapeutically targeted. In IPF, emphasis lies on the role of the pulmonary ROS-producing enzyme NOX4 and the redox-sensitive Src kinase family. Additionally, we study the effect of the only two FDA-approved anti-IPF drugs on the market hereon, as well as new treatment options including kinase inhibitors and dietary antioxidants, supported by comprehensive genomic and gene expression profiling of IPF lungs. Finally, we examine the potential use of volatile organic compounds (VOCs) as biomarkers of disease diagnosis, progression and response to therapy in both in vitro systems and in vivo in naïve patients suffering from IPF and other ILDs.
Extracellular matrix (ECM)
We have identified an important role for epithelial cells in ECM remodeling in vitro, in animal models and in COPD patients. The contribution of this remodeled ECM to the hostile micro-environment to lung repair in emphysema is currently being examined. Of particular attention are the contribution of elastin degradation and its modulation by vitamin K, and the shifted metabolism of the glycosaminoglycan hyaluronan.
Extra-cellular vesicles
In another (related) research line, we investigate the role of secreted factors, particularly extracellular vesicles (EVs), in mediating lung inflammation. We have demonstrated that cigarette smoke extract (CSE) induces increased release of EVs as well as changes in their proteomic composition. Specifically, CSE-induced EVs were enriched in proteins related to hemostasis and could promote activation of coagulation factor X and thrombingeneration. Like mitochondrial dysfunction, induction of procoagulant EVs was mediated by reactive aldehydes and furthermore preventable by antioxidants such as glutathione. Currently, we work with patient materials to investigate whether and how procoagulant EVs contribute to lung inflammation and comorbid cardiovascular disease in patients with COPD. Finally, we showed that EVs contribute to innate immunity during infections with respiratory pathogens and are currently investigating whether this defense mechanism is impaired in COPD.
Early origins of lung disease
Importantly, aberrant lung outcomes faced in later life can have their origins already before birth. Therefore, in a clinically-relevant large animal model of chorioamnionitis, we are examining the impact of BPD on lungs on the long term, and the therapeutic potential of stem cells. We recently demonstrated negative effects of chorioamnionitis on lung resident epithelial progenitor cells and are now examining whether their progenitor functions can be preserved using stem cells.
Scientific impact/Research quality
The scientific quality of the research is apparent from publications in relevant and high impact journals (see below selection of papers), as well as research grants from NWO/ZonMW, National Institute for Public Health and Environment, Dutch Lung Foundation, Wijerhorst Foundation, European Respiratory Society, and Chiesi Pharmaceuticals.
Selection of publications
- Profibrotic epithelial TGF-β1 signaling involves NOX4-mitochondria cross-talk and redox-mediated activation of the tyrosine kinase FYN. Veith C, Hristova M, Danyal K, Habibovic A, Dustin CM, McDonough JE, Vanaudenaerde BM, Kreuter M, Schneider MA, Kahn N, van Schooten FJ, Boots AW, van der Vliet A. Am J Physiol Lung Cell Mol Physiol. 2020 Dec 16
- SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R.EMBO J. 2020;39(10):e105114.
- Redox Imbalance in Idiopathic Pulmonary Fibrosis: A Role for Oxidant Cross-Talk Between NADPH Oxidase Enzymes and Mitochondria. Veith C, Boots AW, Idris M, van Schooten FJ, van der Vliet A. Antioxid Redox Signal. 2019;31(14):1092-1115.
- Proteomic analysis reveals procoagulant properties of cigarette smoke-induced extracellular vesicles. Benedikter, B.J., Bouwman, F. G., Heinzmann, A. C. A., Vajen, T., Mariman, E. C., Wouters, E. F. M., Savelkoul, P. H. M., Koenen, R. R., Rohde, G. G. U., van Oerle, R., Spronk, H. M. & Stassen, F. R. M., 1 Jan 2019, In: Journal of Extracellular Vesicles . 8, 1, 16 p., 1585163.
- Cigarette smoke extract induced exosome release is mediated by depletion of exofacial thiols and can be inhibited by thiol-antioxidants. Benedikter, B. J., Volgers, C., van Eijck, P. H., Wouters, E. F. M., Savelkoul, P. H. M., Reynaert, N. L., Haenen, G. R. M. M., Rohde, G. G. U., Weseler, A. R. & Stassen, F. R. M., Jul 2017, In: Free Radical Biology and Medicine. 108, p. 334-344 11 p
- van de Wetering C, Aboushousha R, Manuel AM, Chia SB, Erickson C, MacPherson MB, van der Velden JL, Anathy V, Dixon AE, Irvin CG, Poynter ME, van der Vliet A, Wouters EFM, Reynaert NL, Janssen-Heininger YMW. Pyruvate Kinase M2 Promotes Expression of Proinflammatory Mediators in House Dust Mite-Induced Allergic Airways Disease. J Immunol. 2020.15;204(4):763-774
- Rutten E, Gopal P, Wouters E, Franssen F, Hageman G, Vanfleteren L, Spruit M, Reynaert N. Various mechanistic pathways representing the ageing process are altered in COPD. Chest. 2016 149(1): 53-61
- P.Gopal, N.L. Reynaert, J.L.J.M.Scheijen, L. Engelen, C.G.Schalkwijk, F.M.E.Franssen, E.F.M. Wouters, E.P.A. Rutten. Plasma AGEs and skin autofluorescence are increased in COPD. ERJ 2014; 43(2): 430-438.
- Ine Kuipers, Catherine Moermans, Renaud Louis, Mieke A Dentener,Yvonne Charles Irvin, Christopher Brightling, Yvonne MW Janssen-Heininger, Emiel FM Wouters, Niki L Reynaert. Increased glutaredoxin 1 and decreased protein S- glutathionylation in sputum of asthmatics. ERJ 2013;41(2):469-72.
- Leermakers PA, Remels AHV, Langen RCJ, Schols AMWJ, Gosker HR. Pulmonary inflammation-induced alterations in key regulators of mitophagy and mitochondrial biogenesis in murine skeletal muscle. BMC Pulm Med 2020;20(1):20
- Aghapour M, Remels AHV, Pouwels SD, Bruder D, Hiemstra PS, Cloonan SM, Heijink IH. Mitochondria: at the crossroads of regulating lung epithelial cell function in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2020;318(1):L149-L164
Users and collaborations
We work closely with a large number of local, (inter)national, governmental and academic partners. State-of-the art in vitro models are developed in collaboration with the MERLN institute (Maastricht University; Prof. Truckenmuller), Leiden University, Groningen University, the Hubrecht Institute, and University of Pittsburgh. For exposure studies, we work with the Dutch National Institute for Public Health and the Environment (RIVM), the Netherlands Organisation for applied scientific research (TNO), the Environmental Protection Agency (USA), University of Louisville, Purdue University and Brown University, and for regulatory implications with the RIVM, the Netherlands Food and Consumer Product Safety Authority (NVWA) and the Study group Tobacco Regulation of the WHO. Studies into redox biology and animal models of asthma are performed within the context of our long-standing collaboration with Prof. Janssen-Heininger and Prof. van der Vliet (University of Vermont, USA).
Early origins of lung diseases are studied together with the Dept of Pediatrics at Maastricht University (Prof. Kramer/Dr. Wolfs). Research on extracellular vesicles and models of viral and bacterial infections are performed in close collaboration with the extracellular vesicle core facility of Philipps University Marburg, Germany. In addition to our internal collaborations with the clinical staff of the Depts of Respiratory Medicine and Pathology at the MUMC, and with Prof, Spruit/Dr. Franssen at the Pulmonary rehabilitation center CIRO, clinical centers of expertise provide patient materials and data (St Antonius Hospital Nieuwegein, Erasmus University Rotterdam, University of Liege (Belgium), KU Leuven (Belgium), University of Frankfurt (Germany), INSERM (France), Thorax Klinik Heidelberg (Germany)). BigCaT and the Norwegian University of Science and Technology (Norway), support our research with expertise in bio-informatics and systems biology. The PLUC facility provides cells with a passport and other lung tissue specimens for research to Maastro, Dept of Toxicogenomics, MERLN and the RIVM.
