Maastricht University Animal Ethics Committee
As of December 2014, the amended Experiments on Animals Act (Wod) has entered into force. This has extended the role of the Animal Ethics Committees (DECs) from only reviewing proposals ethically to also reviewing them scientifically. The DEC-UM provides the Central Dutch Authority for Scientific Procedures on Animals (CCD) with formal advice upon request. For more information about the application procedure for a project license authorisation please visit the website of the CCD. Only the Central Dutch Authority (CCD) can grant licenses for scientific project proposals involving animal testing.
Annual reports / Jaarverslagen
Consulting process DEC-UM
1. The DEC-UM receives a formal request for advice for a (an amendment or extension to a previous) submitted project proposal.
2. The DEC-UM reviews the application in the upcoming plenary meeting.
3. The DEC-UM may have further inquiries with regard to the project proposal, which can be answered by the researchers in writing or in person (upon request by the DEC-UM.
4. If the DEC-UM questions have been answered sufficiently by the researcher(s), the DEC-UM will proceed to draft an advice for the CCD. If not, an additional round of questions will follow.
5. The DEC-UM submits its final advice and most recently received project proposal documents from the researcher(s) to the CCD. The researcher(s) will receive a notification from the DEC-UM (without the proposed advice to the DEC-UM). The role of the DEC-UM ends here.
6. The CCD decides if a license will be granted and will inform both the the mandated license holder of the UM and the researcher(s) about the final decision. The proposed advice of the DEC-UM will be sent along with the decision of the CCD.
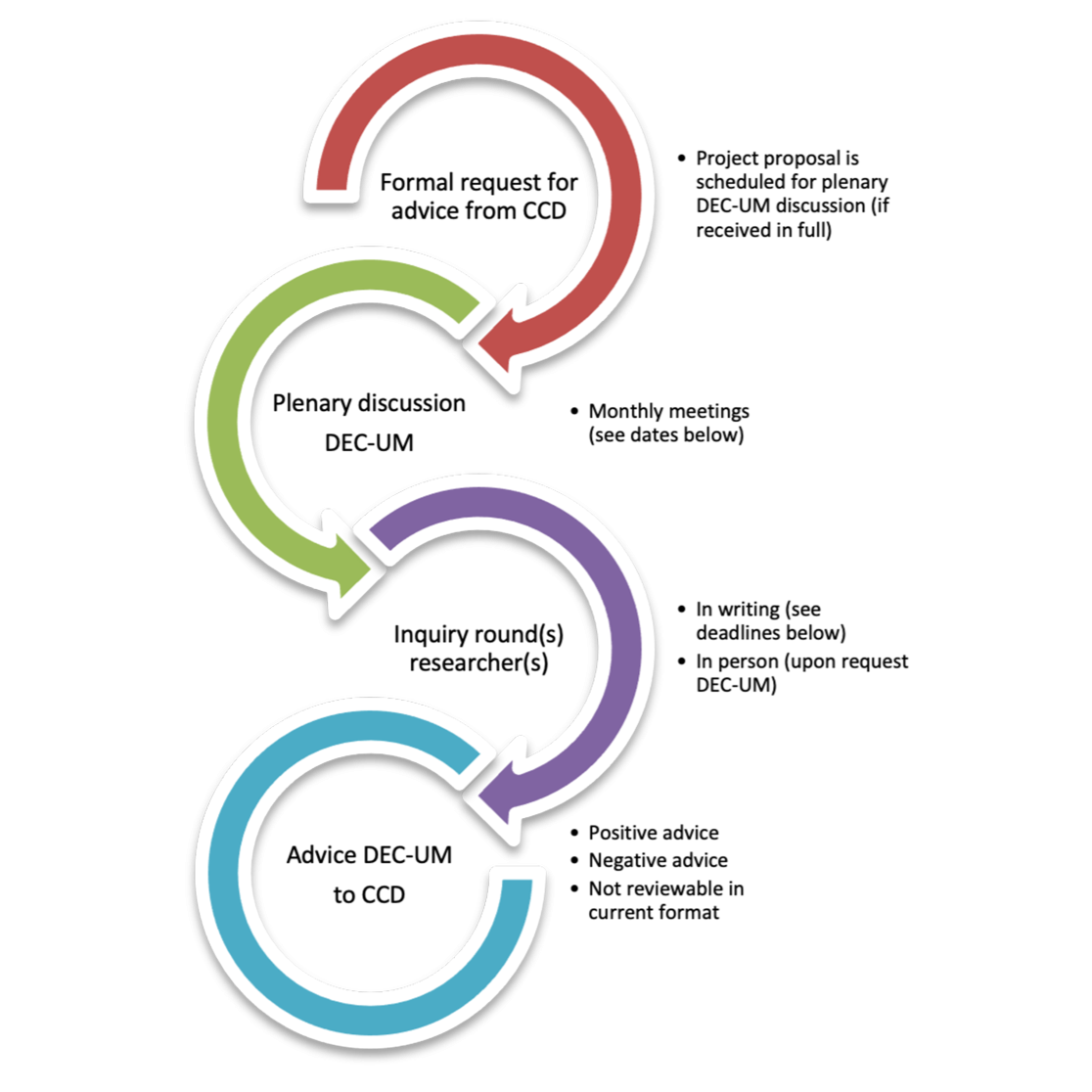
Processing time
The legal processing time for granting a license to a submitted project proposal is 40 working days, which are equally divided between the DEC and the CCD unless it involves a ‘complex submission’. Upon receivement of the formal request for advice from the CCD along with the submitted project proposal documents (i.e. submission form, project proposal, appendice(s) and non-technical summary), the legal processing time for the DEC-UM to produce an advice will commence.
DEC-UM deadlines for researcher inquiry round(s)
The DEC-UM may have some questions with regard to the submitted project proposal, which may affect their proposed advice to the CCD. Therefore, the researcher(s) are given the opportunity to clarify their project proposals. If requested to be delivered in writing, the researcher is asked to uphold the following delivery deadlines:
| Plenary meetings DEC-UM | Delivery deadlines for researcher(s): (no later than 23:59 hours) |
|---|---|
| 23 January 2026 | 15 January 2026 |
| 27 February 2026 | 19 February 2026 |
| 27 March 2026 | 19 March 2026 |
| 24 April 2026 | 16 April 2026 |
| 22 May 2026 | 14 May 2026 |
| 19 June 2026 | 11 June 2026 |
| 10 July 2026 | 2 July 2026 |
| 28 August 2026 | 20 August 2026 |
| 25 September 2026 | 17 September 2026 |
| 30 October 2026 | 22 October 2026 |
| 20 November 2026 | 12 November 2026 |
| 11 December 2026 | 3 December 2026 |
Composition DEC-UM on 1 January 2024
| Members | Scientific focus | Expertise | Involved in animal research | Affiliated to the license holder |
|---|---|---|---|---|
| Member 1 (chair) | Veterinary medicine | VM;SA;D;P;HC | I-NL | Not affiliated |
| Member 2 | Cardiology | SA;D | Involved | Affiliated |
| Member 3 (vice-chair) | Oncology | SA;D;P | Involved | Affiliated |
| Member 4 | Neurology | D;SA;HC | Involved | Affiliated |
| Member 5 | Pulmonology | SA;D | Not involved | Not affiliated |
| Member 6 | Ethics | E;SA;D;HC | Not involved | Not affiliated |
| Member 7 | Biomaterials | SA;D;HC | Involved | Not affiliated |
| Member 8 | Pharmacology/Toxicology | SA;D;HC | Not involved | Not affiliated |
| Advisor | Chair Animal Welfare Body (IvD) | Involved | Affiliated | |
| Secretary | Not involved | Affiliated |
Abbreviations: SA = the scientific research areas and applications implementing animal testing, taking into account replacement, reduction and refinement on the scientific focus; D = design of animal tests, including statistical aspects; VM = veterinary medicine practice in context of research or wild-life animals; HC = housing and caring of animals applied in research; E = ethics; P = protection of research animals; NI = not involved in animal testing; NI-NL = not involved in animal testing within the Netherlands;