Enterohepatic cycle disturbances in surgical patients
Division 2: Liver and Digestive Health
Department of Surgery
Background
What are the consequences of disturbances in the enterohepatic circulation?
Bile salts are the prototypical signaling molecules involved in bidirectional communication in the gut-liver axis. Synthesized in the liver, bile salts are secreted in the biliary network for eventual release in the proximal small intestine to aid in digestion and absorption of dietary lipids. Active reclamation of the bulk of bile salts in the terminal ileum along with passive re-uptake of bile salts spilling over into the colon, allow near-complete conservation and return of bile salts to the liver via the portal blood for re-secretion into bile. During their enterohepatic cycle (EHC), bile salts activate dedicated plasma membrane (e.g. TGR5) and nuclear bile salt receptors (e.g. FXR) in the small intestine and liver. Attendant signaling actions are pivotal for maintaining metabolic homeostasis, liver tissue homeostasis, gut barrier integrity and inflammatory control in the intestine and liver (Figures 1 and 2).
Figure 1: Enterophepatic actions of FXR
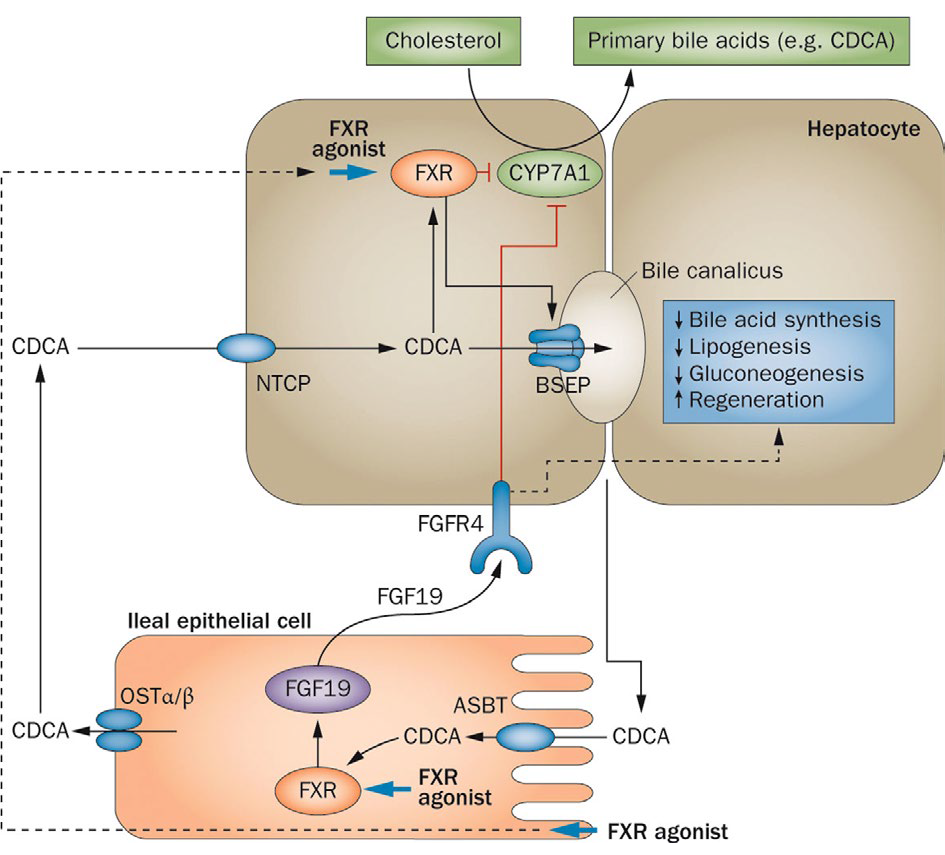
Bile acids (exemplified as the primary bile acid CDCA) are produced in the liver by CYP7A1-initiated conversion of cholesterol in primary bile acids. Bile salts are secreted via BSEP into the canalicular lumen. In the ileum, bile salts are reabsorbed via ASBT in terminal ileum enterocytes. Here, they bind and activate FXR and this stimulates the transcription of FGF19, which encodes a protein that is secreted into the portal circulation. In the liver, FGF19 binds to its receptor FGFR4, which activates a signalling pathway involving MAP kinases and causes repression of CYP7A1, thus downregulating bile acid synthesis. After OSTa/13- mediated secretion into the portal circulation, bile acids are taken up by the liver via NTCP, thus, completing the enterohepatic cycle. In the liver, bile acids bind to FXR, which transcriptionally upregulates a protein called SHP (not shown) that interferes with expression of CYP7A1. Oral FXR agonists will affect FXR in both liver and intestine and this strongly downregulates CYP7A1 both by FGF19-dependent and FGF19-independent effects. FGF19 additionally affects lipogenesis, gluconeogenesis and liver regeneration. Abbreviations: ASBT, apical sodium-dependent bile salt transporter; BSEP, bile salt export pump; CDCA, chenodeoxycholic acid; CYP7A1, cholesterol 7-a-monooxygenase; FGF19, fibroblast growth factor 19; FGFR4, fibroblast growth factor receptor 4; FXR, farnesoid X receptor; NTCP Nattaurocholate cotransporting polypeptide; OST, organic solute transporter; SHP, small heterodimer partner.
Figure 2: TGR5-expressing tissues and targets
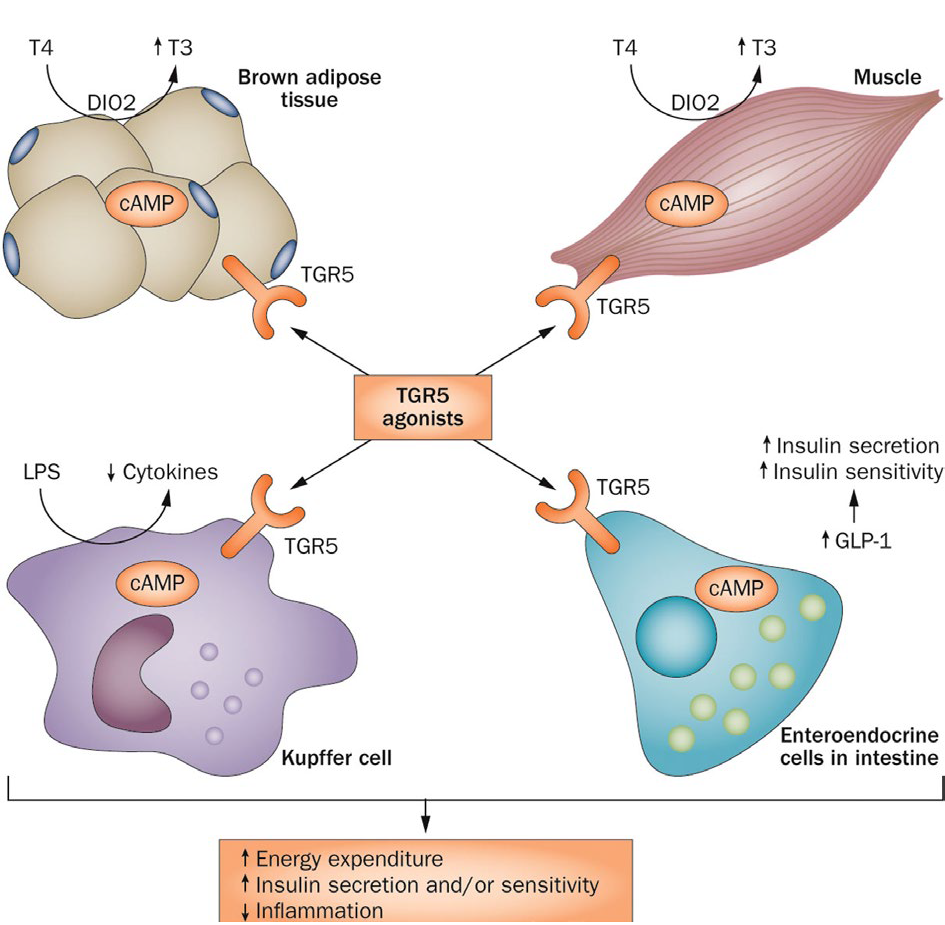
TGR5 signalling in skeletal muscle and brown adipose tissue results in local activation of the deiodinase D102 that generates active thyroid hormone (13), an important regulator of metabolism and energy homeostasis. Bile acids in the intestinal lumen activate TGR5 in enteroendocrine cells, resulting in release of the incretin GLP-1. In Kupffer cells and macrophages, TGR5 activation inhibits LPS-induced cytokine production. Abbreviations: D102, type II iodothyronine deiodinase; GLP-1, glucagon-like peptide 1; LPS, lipopolysaccharide; 13, active thyroid hormone; 14, inactive thyroxine; TGR5, transmembrane G protein-coupled receptor TGR5 (also known as GPBAR1).
Disturbances in the EHC result in a loss of these signaling actions, as well as loss of physicochemical actions of bile salts. Consequences of perturbed EHC thus include fat malabsorption and deficiency of fat-soluble vitamins, small intestinal bacterial overgrowth whether or not accompanied by (portal) endotoxemia and hepatic bile salt accumulation and attendant cellular damage and inflammatory sequela. Dysregulated bile salt homeostasis/bile salt toxicity is considered an important etiological factor in liver disease in patients with EHC disturbances. Likewise, it is linked to impaired liver regeneration and post-operative complications in (post)cholestatic patients with hepatobiliary tumors.
What are causes of abrogated enterohepatic circulation?
Clinical scenarios giving rise to defective EHC include obstruction caused by compression of the bile ducts by a tumor mass inside (e.g. perihilar cholangiocarcinoma) or outside (e.g. head of pancreas carcinoma) the liver. This results in impaired flow of bile (i.e. cholestasis) towards the duodenum, hepatic retention and accumulation of bile salts and intestinal bile salt deficiency. In surgical patients with a proximal enterostomy or enterocutaneous fistula, bile and other digestive fluids are lost via the stomal or fistula output. This lack of intestinal continuity can give rise to intestinal failure-associated liver disease. This syndrome also occurs as a consequence of intestinal failure caused by massive resection of intestine resulting from a surgical emergencies. In patients with critical illness, gallbladder dysfunction is a common feature, with impaired expulsion of bile resulting in EHC perturbation. Likewise, patients receiving (total) parenteral nutrition lack the enteral stimuli that induce gallbladder contraction and enterohepatic bile salt cycling.
Figure 3: Kaplan-Meier curves for patients with low, intermediate, and high MESIF scores.
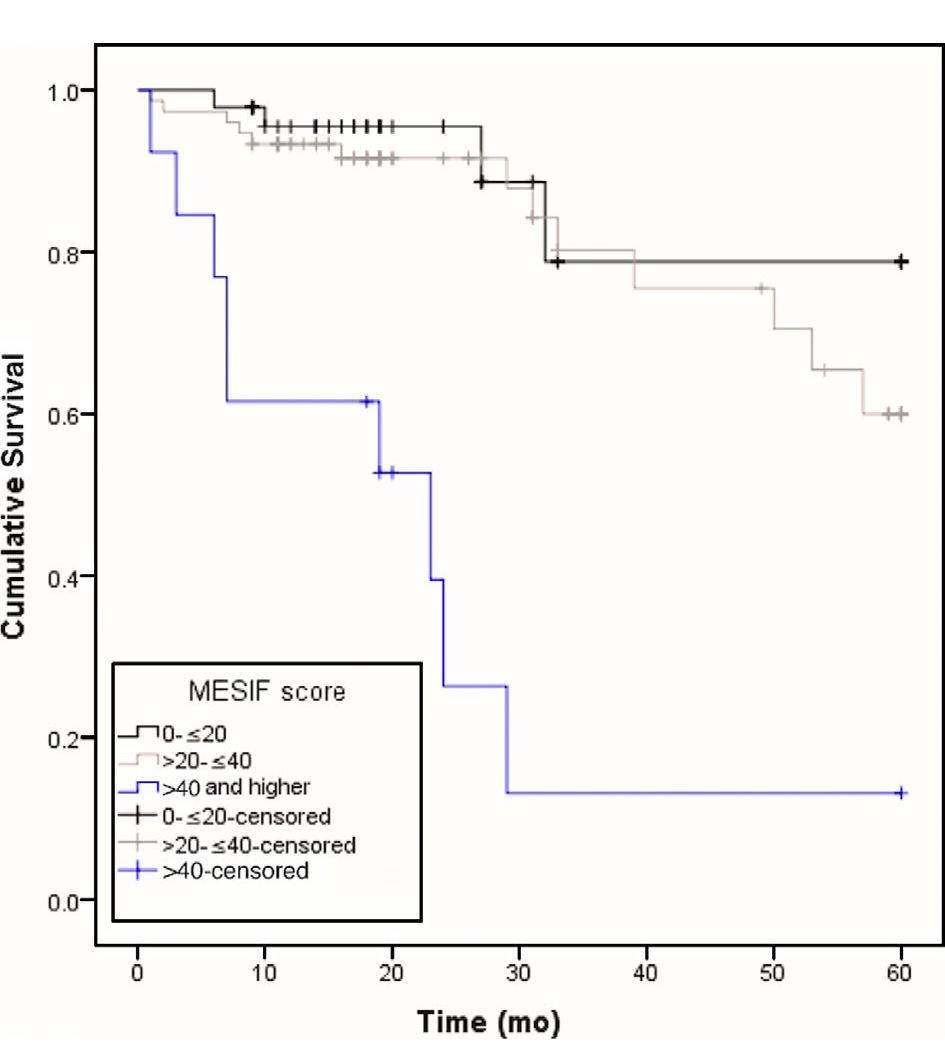
Patients with high MESIF scores (> 40) had a significantly lower 5-y survival rate than patients with low (scores between 0 and 20) or intermediate (scores between 20 and 40) MESIF scores (log-rank test, P < 0.0001). MESIF, Model for End-Stage Intestinal Failure.
Major breakthroughs
Low FGF19 levels predict poor survival in adult patients with chronic intestinal failure.
FGF19 is an enterokine that is transcriptionally induced following uptake of bile salts from the intestinal lumen, and mediates negative feedback regulation of bile salt synthesis (Figure 1). Low levels of FGF19 were found in adult patients with chronic intestinal failure, and this correlated with chronic cholestasis and poor survival (Figure 3). Low FGF19 is one of the three predictors incorporated in a risk model of survival termed Model for End-Stage Intestinal Failure (MESIF). The MESIF score may help to identify patients for closer clinical monitoring or earlier referral to intestinal transplantation centers. https://pubmed.ncbi.nlm.nih.gov/31075790
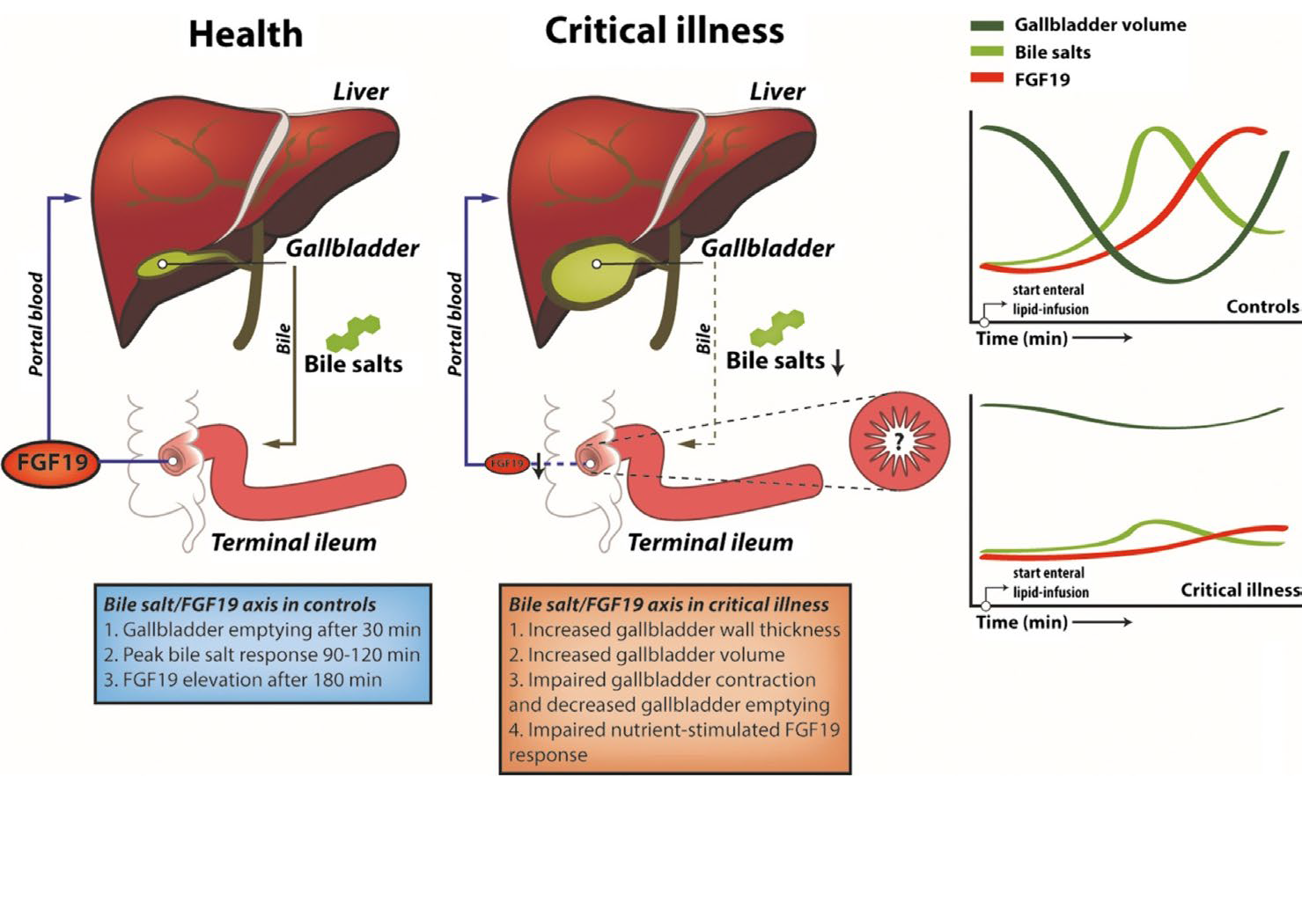
The nutrient-stimulated FGF19 response is abrogated in critically ill patients.
Enteral lipids induce gallbladder emptying and postprandial elevation of the metabolic hormone FGF19. The nutrient-stimulated FGF19 response is impaired in ICU patients (Figure 4, below), which is mechanistically linked to gallbladder dysmotility in critical illness. This may contribute to disturbed liver metabolism in these patients and has potential as a nutritional biomarker. https://pubmed.ncbi.nlm.nih.gov/30933374
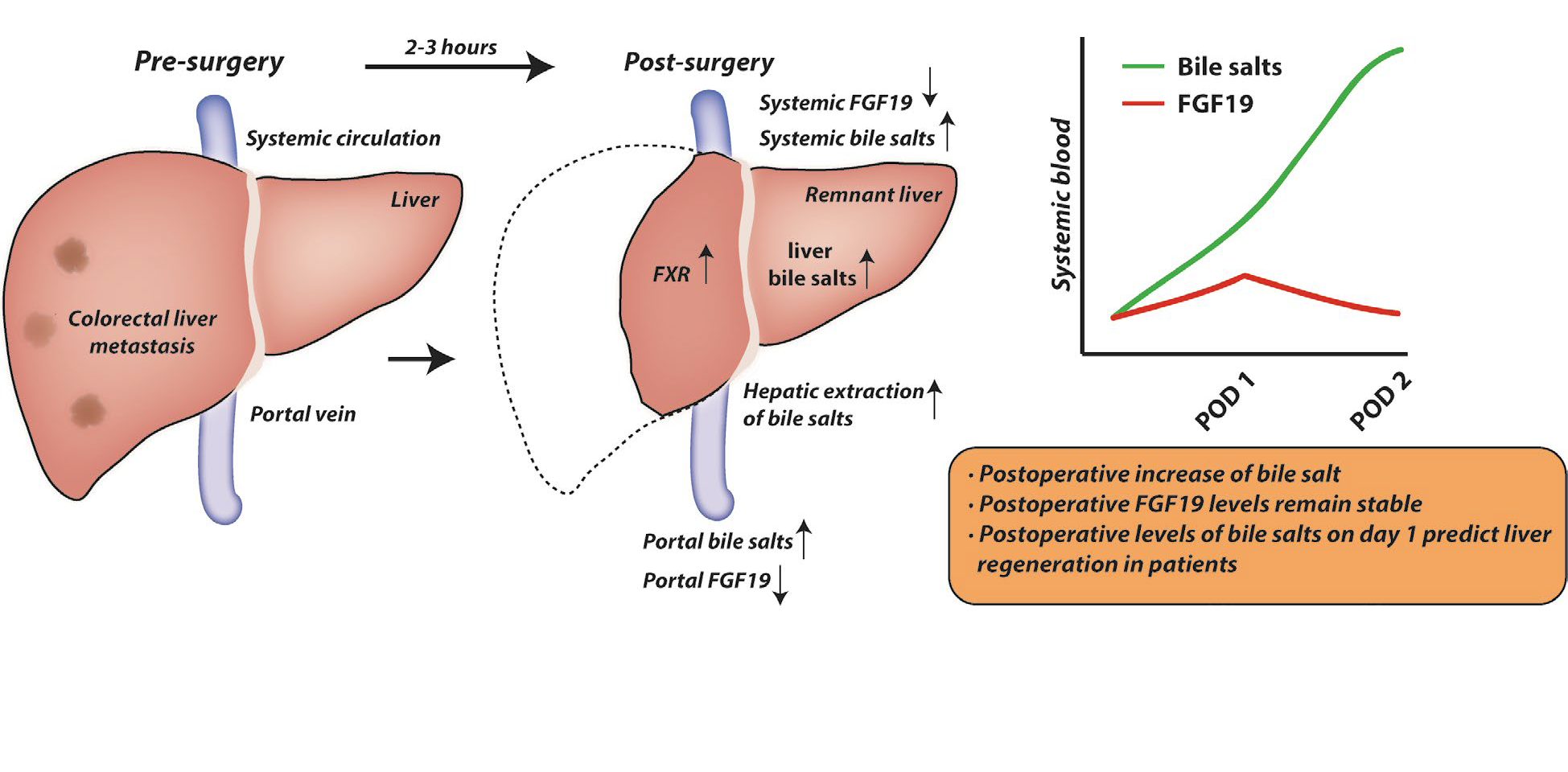
Bile salt levels at post-operative day 1 predict liver regeneration in humans.
In patients undergoing liver resection for colorectal liver metastasis (CRLM), levels of bile salts in the portal circulation were elevated 2-3 hrs after start of liver transection. This was accompanied by elevated hepatic bile salt content and associated with transcriptional induction of genes engaged in priming hepatocyte cell cycle re-entry. Bile salt levels increased in the postoperative trajectory, and levels at post-operative day 1 predicted liver regeneration in these patients (Figure 5, below). This observation was replicated in an external validation cohort. Strikingly, a divergent postoperative bile salt course was observed in (post)cholestatic patients undergoing resection for perihilar cholangiocarcinoma, in which postoperative complications are far more frequent than in patients with CRLM.
Chyme reinfusion restores the regulatory bile salt-FGF19 axis in intestinal failure patients.
Automated chyme reinfusion (CR) in intestinal failure patients with a temporary double enterostomy restores intestinal function and protects against liver injury. CR evoked an increase in plasma FGF19 and decreased C4 levels, indicating restored regulation of bile salt synthesis via endocrine FGF19 action (Figure 6, below). Furthermore, citrulline and albumin levels were gradually rising after CR, while abnormal serum liver tests normalized after CR, indicating restored intestinal function, improved nutritional status and amelioration of liver injury. Beneficial effects of chyme reinfusion are partly mediated by recovery of the bile salt-FGF19 axis and subsequent homeostatic regulation of bile salt synthesis.
Figure 6: Automated chyme reinfusion
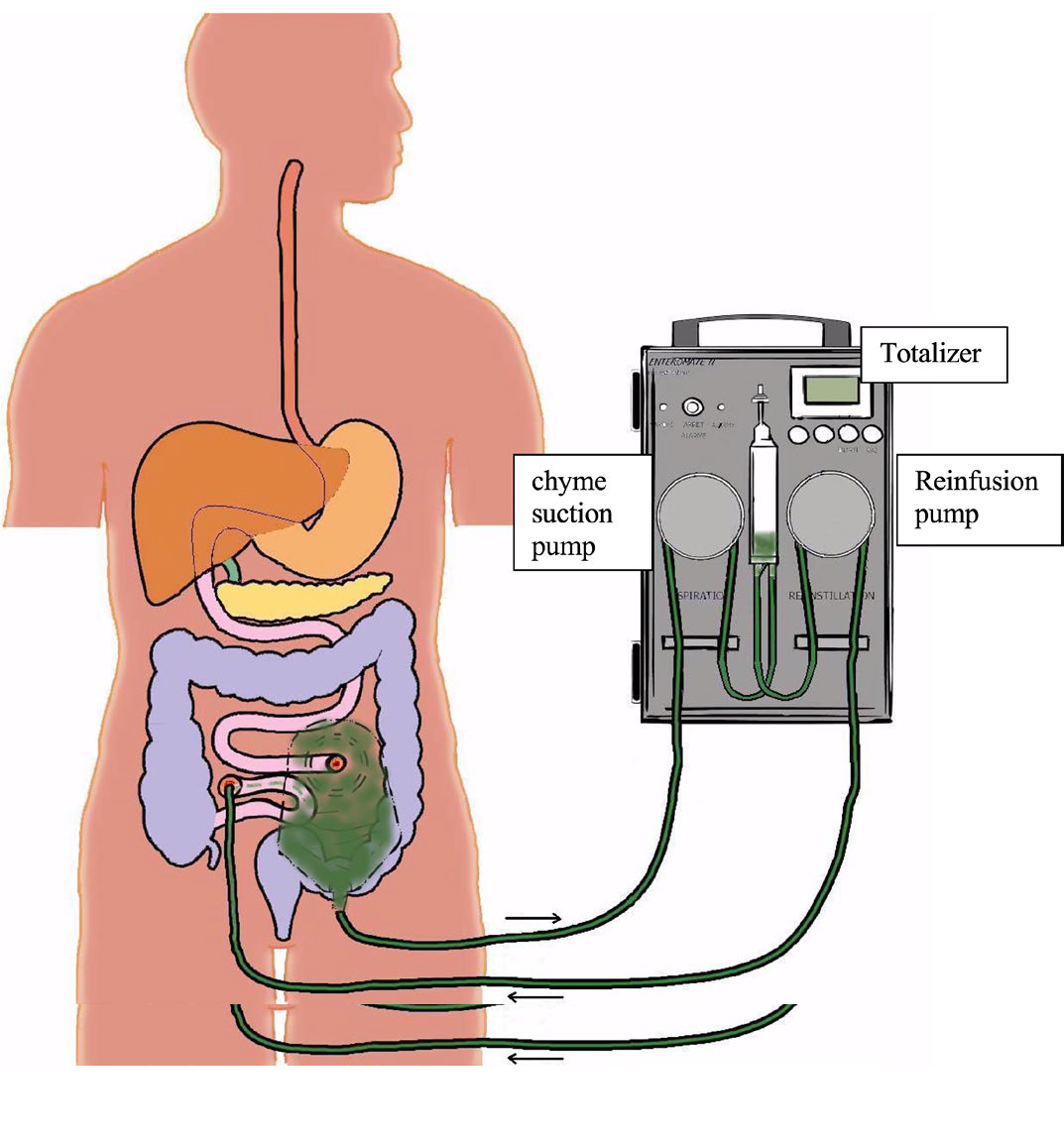
Figure 6: Automated chyme reinfusion in intestinal failure patients with a temporary double enterostomy restores intestinal function and protects against liver injury via increased plasma FGF19 and decreased C4 levels, indicating restored regulation of bile salt synthesis.
Users and collaborations
We collaborate locally with Prof. Ron Heeren (M4i) and Dr. Rob Vreeken (M4i) to study spatial localization of bile salts and sulfatides in cholangiopathies, and with Dr. John Penders, Dept. of Medical Microbiology to investigate the interaction between the gut microbiota and bile salts.
National collaborations exist with Dr. Barbara de Koning, Erasmus UMC, Rotterdam (pediatric intestinal failure) and Dr. Geert Wanten, Radboud UMC, Nijmegen, with whom we study acute and chronic intestinal failure in the pediatric and adult population. In collaboration with Dr. Maarten Soeters, Amsterdam UMC we study metabolic consequences of enterohepatic bile salt signaling in human subjects.
International collaborations
With Prof. Mathias Hornef, RWTH Aachen, Aachen, Germany we study the interaction between the gut microbiota and bile salts. Bile salt receptor-based enhancement of liver regeneration in mouse is studied in collaboration with Prof. Isabelle Leclercq, Université Catholique de Louvain, Brussels, Belgium. In a long-standing collaboration, dysregulation of the bile salt/FGF19 regulatory axis and other aspects of bile salt (patho) physiology are studied in patient populations together with Dr. Martin Lenicek, Charles University Prague, Czech Republic. Joint studies with Dr. Espen Melum, Oslo University Hospital/Norwegian PSC Research Center, Oslo, Norway, focus on gaining insight into the role of sulfatides in cholangiocyte biology and the bile duct disorder primary sclerosing cholangitis. Together with Prof. Ronan Thibault, Rennes University Hospital, France, we study adult patients with acute intestinal failure, receiving chyme reinfusion therapy.
We are also embedded within the prestigious Sonder-forschungsbereich 1382 “Die Darm-Leber-Achse-Funktionelle Zusamenhange und Therapeutische Strategie” of the Deutsche Forschunsgemeinschaft, where we are collaboration partners in 7 of the 16 projects.
Industrial partners
The effects of an FDA-approved FXR agonist on liver growth after experimental portal vein embolization are studied in collaboration with Dr. Luciano Adorini, Intercept Pharmaceuticals Inc.
Who is involved?
The research team is headed by P.I.s Frank Schaap and Steven Olde Damink and at present consists of five PhD students: Kim van Mierlo, Kiran Koelfat (supported by NWO/ESPEN), Lin Cheng & Xinwei Chang (both supported by Chinese Scholarship Council) and Ümran Ay (supported by the German Research Foundation). A visiting scientist (Dr. Martin Lenicek) was previously supported by a NWO travelling grant and is presently working in our team with support of an EU mobility grant. Staff support is provided by technicians Annemarie Bijnen and Bas Boonen (general lab support) and Dr. Hans van Eijk (analytical support). We are also embedded within the prestigious Sonderforschungsbereich 1382 “ Die Darm-Leber-Achse-Funktionelle Zusamenhange und Therapeutische Strategie of the Deutsche Forschunsgemeinschaft as part of a collaboration with the RWTH Aachen.
Scientific impact/Research quality
Our focus on human translational studies is internationally recognized and well appreciated, as mirrored by publications in high impact journals in the field of hepatology and gastroenterology. Most of the knowledge on bile salt signaling stems from mouse studies, with many aspects of bile salt biology differing between man and mouse, these translational studies are of key importance.
Selection of publications
- Koelfat KVK et al. Chyme reinfusion restores the regulatory bile salt-FGF19 axis in intestinal failure patients. Hepatology, revised manuscript under consideration.
- Koelfat KVK et al. Bile salt and FGF19 signaling in the early phase of human liver regeneration. Hepatol. Commun., in press
- Koelfat KVK, Plummer MP, Schaap FG, Lenicek M, Jansen PLM, Deane AM, Olde Damink SWM. Gallbladder Dyskinesia Is Associated With an Impaired Postprandial Fibroblast Growth Factor 19 Response in Critically Ill Patients. Hepatology. 2019;70:308-318.
- Koelfat KVK, Huijbers A, Schaap FG, van Kuijk SMJ, Lenicek M, Soeters MR, Wanten GJA, Olde Damink SWM. Low circulating concentrations of citrulline and FGF19 predict chronic cholestasis and poor survival in adult patients with chronic intestinal failure: development of a Model for End-Stage Intestinal Failure (MESIF risk score). Am J Clin Nutr. 2019;109:1620-1629.
- Schubert K, Olde Damink SWM, von Bergen M, Schaap FG. Interactions between bile salts, gut microbiota, and hepatic innate immunity. Immunol Rev. 2017;279:23-35.
- Olthof PB, Huisman F, Schaap FG, van Lienden KP, Bennink RJ, van Golen RF, Heger M, Verheij J, Jansen PL, Olde Damink SW, van Gulik TM. Effect of obeticholic acid on liver regeneration following portal vein embolization in an experimental model. Br J Surg. 2017;104:590-599.
- Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11:55-67.
- Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228-1235.
Societal impact
Our PhD students have won multiple prizes presenting this original work on different conferences, e.g. the second (2019) and the first (2020) price for best abstract and oral presentation at the ESPEN congresses. Kiran Koelfat also received an ESPEN Fellowship (2018). We consider it very important to communicate our data and insights to fellow researchers and to the broader community, as exemplified from several recent activities. The findings of chyme reinfusion study have been included in the ESPEN guidelines of treatment of Intestinal Failure and is currently being validated in a large multi-center study in France. Yearly lecture on “bile salts & liver disease” are given (by FGS) to Biomedical Sciences student of UM and in the framework of a course (Lipids in health and disease) for 2nd-3rd year medical students at the RWTH Aachen.
Future Perspectives
Future translational research will focus on improving liver regeneration in the (post)cholestatic patient, typically patients with resectable perihilar cholangiocarcinoma. In pre-clinical models we have explored pharmaceutical activation of FXR as a strategy to accelerate liver regeneration in both non-cholestatic, cholestatic, and post-cholestatic animals. While liver regeneration after partial hepatectomy was not enhanced in any of these groups, FXR agonism was effective in accelerating portal vein embolization-induced liver growth in rabbit. Another spear point will be to study the interaction between the gut microbiota, bile salts and host immunity in the context of liver regeneration.