Human Tissue Analysis
Brown adipose tissue (BAT) research: combining Human Tissue Analysis and Imaging
BAT has long been considered non functional during adult life. In mammals, BAT is able to produce heat to prevent hypothermia during cold exposure. Here, BAT metabolizes fatty acids and glucose in order to maintain the body’s core temperature. Interestingly, mammals with dysfunctional BAT become obese and develop type 2 diabetes and dyslipidemia. In man, BAT was known to be present and active shortly after birth, but could not be shown to be active in adult man. However, in 2009 studies from NUTRIM van Marken Lichtenbelt et al. NEJM 2009using metabolic imaging with 18-F-FDG-PET-CT scans have shown adult man possesses active BAT during cold exposure (figure 1). In addition, BAT was negatively related to obesity. Thus, obese people have low or absent BAT. Since active BAT is known to be able to increase energy expenditure, it was suggested activating BAT could be a possible new therapeutic target to treat obesity. After showing BAT is present and active, in 2012 NUTRIM researchers have shown that morbidly obese subjects that have low or absent BAT are able to increase their BAT activity after weight loss induced by bariatric surgery Figure 2 .These results suggest BAT activity can be recruited and further research is currently performed to study possible activators of BAT. BAT is sympathetically innervated and therefore adrenergic stimulation with the sympaticomimeticum isoprenaline was studied, but did not show to be able to induced BAT activity, Figure 3. In addition, vagus nerve stimulation (parasympathetic) was shown to increase energy expenditure with possible involvement of BAT, suggesting direct electrical nerve stimulation could possible increase BAT activity. Other activators of BAT are currently studied; one of the current NUTRIM projects investigates the effect of long-term cold exposure on BAT activity and preliminary results suggest frequent cold exposure significantly increases BAT volume and activity.
Information: Dr. Wouter van Marken Lichtenbelt, Department of Human Biology
Figure: FDG-PET-CT image of lean young adult man after cold exposure showing BAT activity in supraclavicular and paravertebral areas (A). HE-stained slide showing typical multivacuolar brown adipocytes (BAT) and univacuolar white adipocytes (WAT) (B). Immunofluorescence stained slide showing the BAT-unique protein UCP1 that facilitates heat production in BAT (UCP1 in green).
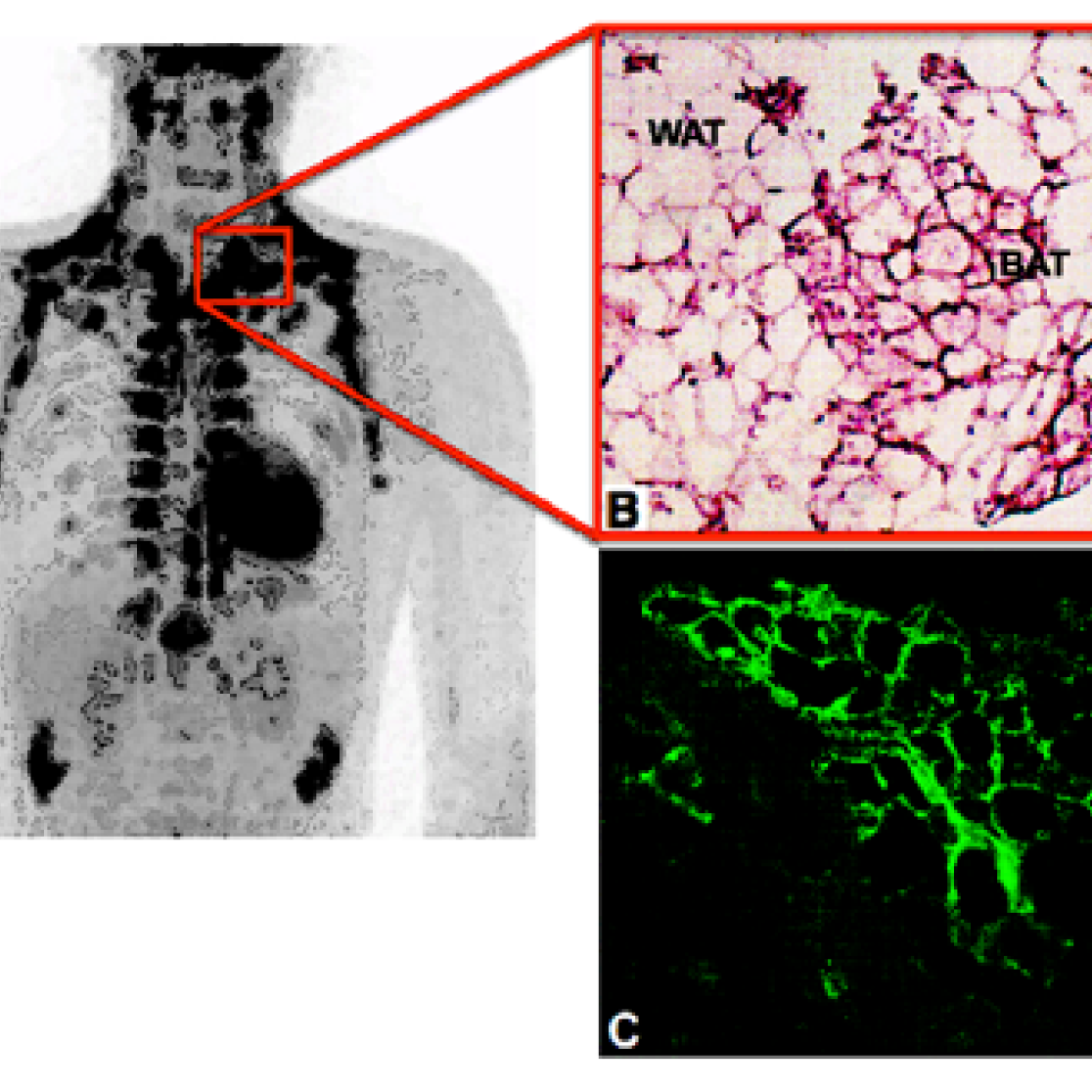
Figure: (A) PET-images of five morbidly obese subjects before bariatric surgery (A) and after weight loss (B). (C) PET-image, CT-image and PET-CT-fusion-image of the subject that showed the largest increase in BAT activity before (C) and after weight loss (D). BAT activity was recruited in both supraclavicular and paravertebral areas, indicated by full and dashed arrows respectively. Full arrows indicate bilateral supraclavicular regions where BAT activity was observed after weight loss. Dashed arrows indicate bilateral paravertebral regions where BAT activity was observed after weight loss.
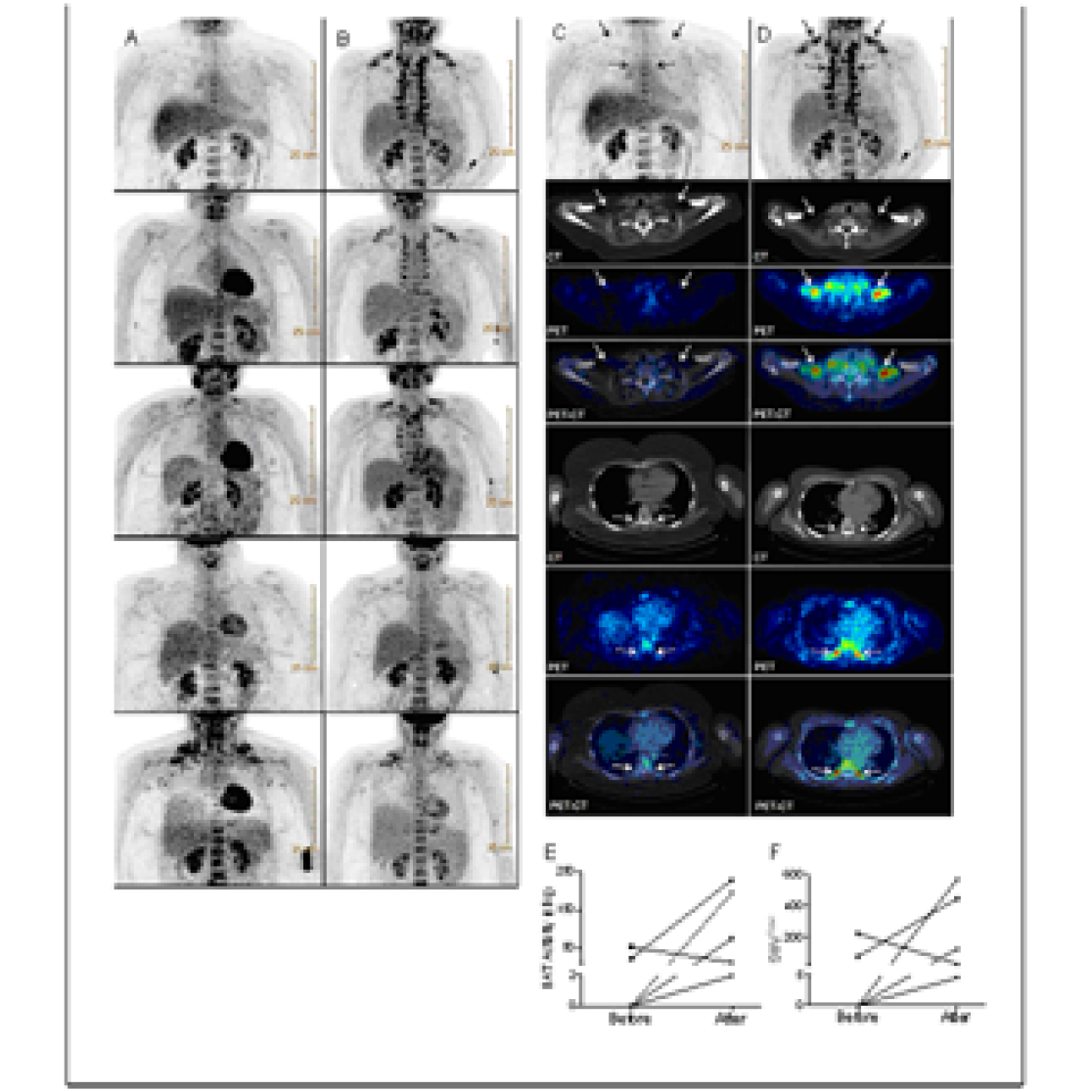
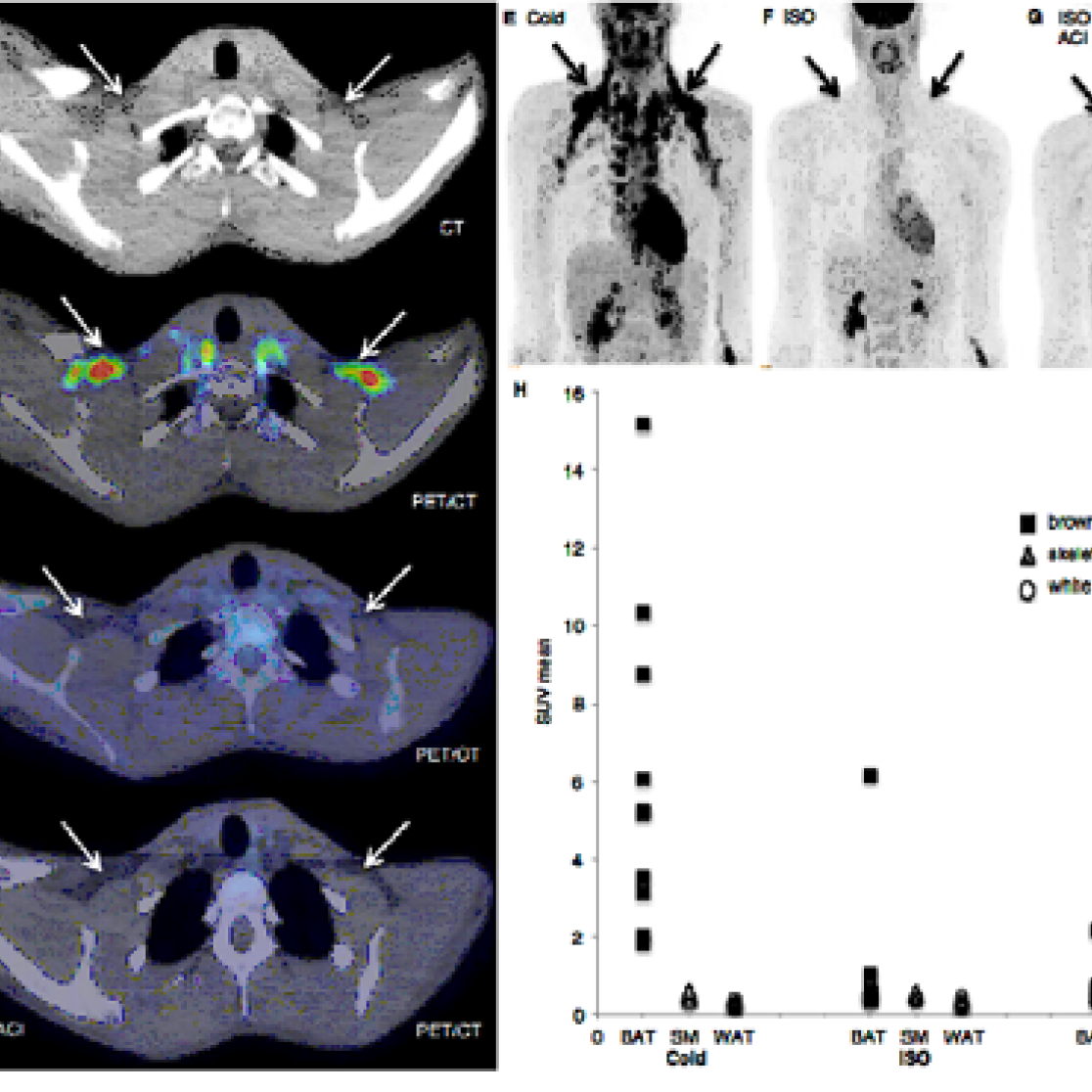
Figure: Brown adipose tissue activity during ISO infusion and cold exposure (A) CT image of the supraclavicular area. (B) Cold exposure [18F]FDG-PET/CT image showing cold-activated BAT. (C) ISO [18F]FDG-PET/CT image showing no BAT activity during ISO. (D) ISO and acipimox [18F]FDG-PET/CT image showing no effect on [18F]FDG-uptake in supraclavicular BAT. (E) Cold exposure [18F]FDG-PET/CT image of the upper body showing BAT activity in the neck, supraclavicular, paraspinal, para-aortic, axillary, mediastinal, and perirenal regions. (F) ISO [18F]FDG-PET/CT image of showing no BAT activity during isoprenaline. (G) ISO and acipimox [18F]FDG-PET/CT image showing no effect on [18F]FDG-uptake in BAT locations. (H) SUV mean uptake in tissues, ISO (n=10) ISO+ACI (n=5).
Immunological research
The research within the immunology-oriented lab aims at unraveling the role of innate immunity and inflammation in situations of compromised gut and liver function in a translational research setting. Basic, translational, and clinical studies are performed on several topics including ischemia-reperfusion, sepsis, obesity, non alcoholic steatohepatitis, and parenteral nutrition. In addition, novel assays to evaluate intestinal function and integrity are developed and validated.
Tissue sampling during surgery enables the study of human intestinal and liver pathology. Available techniques are RT-PCR, cloning, ELISA, Western blotting, in vitro cell culture, immunohistochemistry, and fluorescence microscopy. Furthermore, the knowledge and equipment is available to generate high-quality polyclonal and monoclonal antibodies (hybridoma technology) and to develop ELISA’s to detect markers of inflammation and organ injury in biological samples.
Information: Dr. Kaatje Lenaerts, Department of Surgery
In-Vitro research
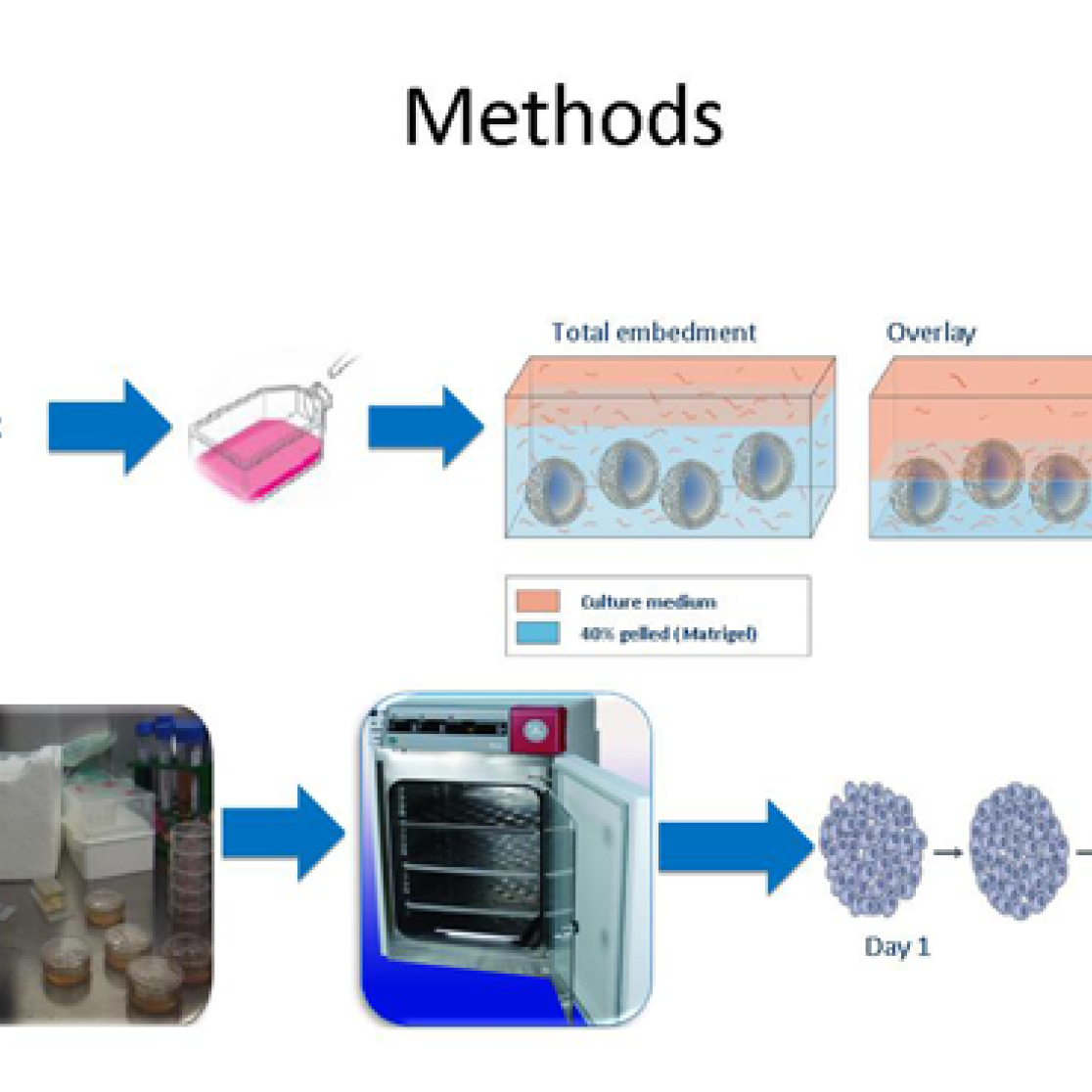
3D cell culture. In this model intestinal epithelial cells form a 3-dimensional spheres structure, enabling the investigation of nutritional components on intestinal barrier function.
Information: Dr. Fred Troost, Department of Internal Medicine
In-Vivo research
Over the last 30 years, the metabolic research of the department has matured from 'black box' biochemical research to innovative in vivo inter-organ amino acid and protein metabolism, facilitating a great number of PhD studies and gaining national and international recognition. New analytical approaches including mass spectrometry have been introduced among others to facilitate stable isotopes tracing. More recently, techniques have been developed to probe gut permeability and short chain fatty acid metabolism.
Three fully automated liquid chromatography mass spectrometry systems are housed within a climatised laboratory, together with 4 stand-alone HPLC systems and surrounding equipment. The facility is operated using a high degree of automation, enabling maximal output with minimal personnel involvement and has an open attitude to support research from collaborating groups within NUTRIM, and the MUMC+ as well as from outside this organization.
Information: Dr. Hans van Eijk, Department of Surgery
