Human Intervention Studies
Gluten intolerance is a wide spread condition causing a variety of gastro-intestinal complaints upon the consumption of gluten. In its most severe case, one speaks of celiac disease. Besides a lifelong gluten free diet, no treatment for this disorder exists. Gluten are highly resistant to digestive breakdown in our gastro-intestinal tract, and resulting gluten peptides can trigger immunological reactions.
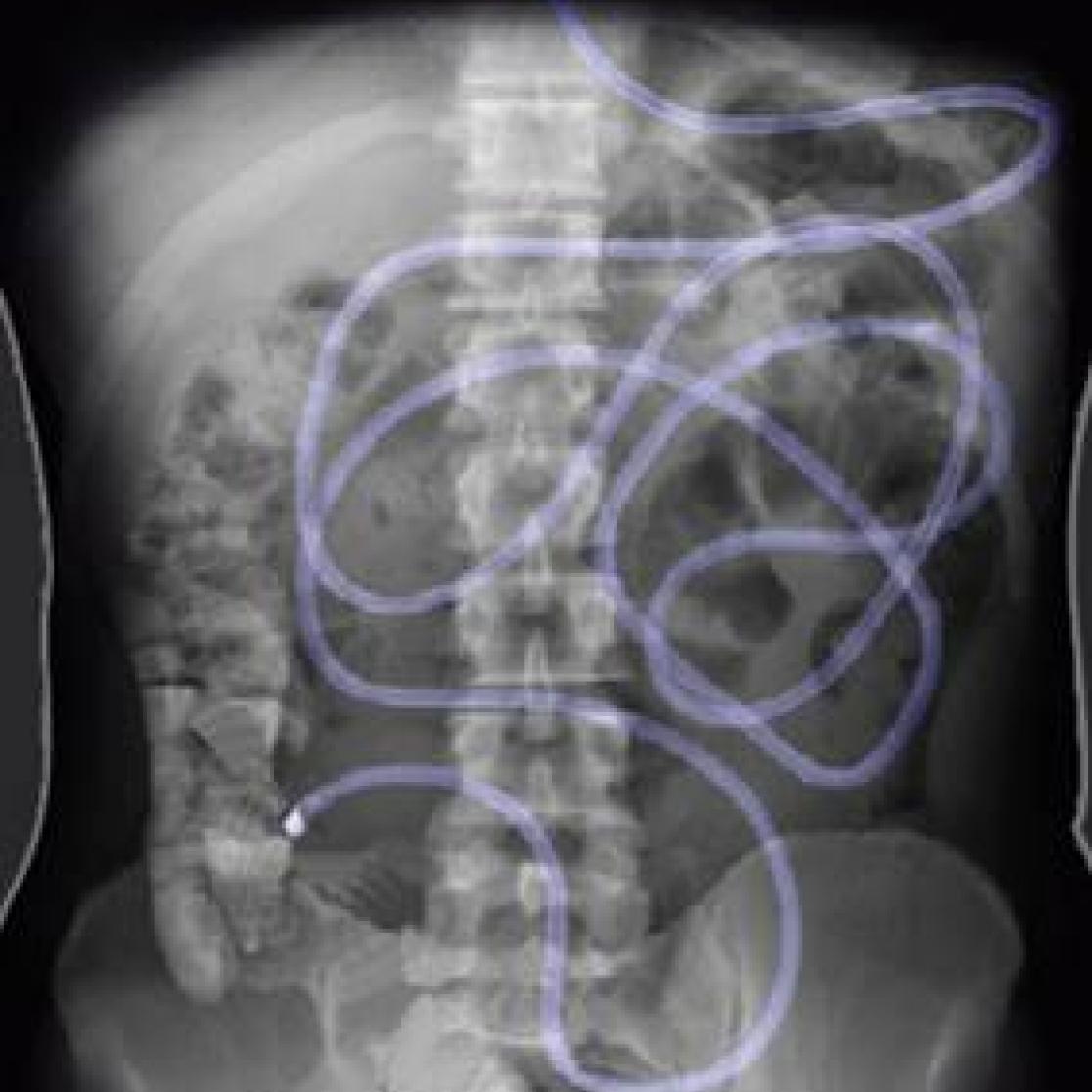
In a human intervention study with healthy volunteers a food-grade enzyme on the digestion of gluten in the stomach and the appearance of gluten peptides in the duodenum is investigated. A triple-lumen feeding cathether is inserted under intermittent fluoroscopic control, with the tube tip located in the duodenum. This catheter is used to administer test meals and enzyme directly into the stomach, and for the aspiration of fluid samples from the stomach and the duodenum.
Further, an inert dilution marker is continuously perfused into the proximal duodenum, to correct for gastrointestinal secretions. Throughout the experiment, blood samples are taken to determine the gastric emptying rate via measuring the recovery of ingested acetaminophen.
Information: Dr. Fred Troost, Department of Internal medicine
Gut microbiota as an important factor in fat distribution, insulin sensitivity and glucose and lipid metabolism
The intestinal microbiota could play an important role in the development of obesity and type 2 diabetes mellitus. The role of gut-derived short-chain fatty acids (SCFA), the formation of which is enhanced by microbial fermentation of fibre, is still controversial.
In a placebo controlled, double-blind, randomized crossover pilot study we will validate in 8 overweight/obese (BMI ≥ 25kg/m2 ≤ 34.9kg/m2) healthy male volunteers whether rectal administration of SCFA is a good model for studying the acute metabolic effects of SCFA. For this, it will be investigated if site of administration (in distal or proximal colon) of SCFA differentially affects parameters of substrate and energy metabolism and to test the duration of short-term effects of SCFA administration.
All subjects are studied two times, with once infusions in the proximal colon and once infusions in the distal colon, each with three different infusions in randomized order consisting of a low concentration of SCFA, a high concentration of SCFA and a placebo. The infusions will take place via a catheter inserted with a colonoscopy, which will be clipped inside the proximal or distal colon for three consecutive days.
Information: Dr. Fred Troost, Department of Internal Medicine
Ileal brake
The appearance of intact nutrients into the first part of the small intestine or the last part of the small intestine, during a meal and during the postprandial phase results in a feed-back from different parts of the intestine to the stomach, to the small intestine and to the central nervous system. All these processes inhibit, in concert, food processing in the gastrointestinal tract, satiation and appetite sensations and, consequently, food intake. These processes are involved in the so-called intestinal brake. The location at which the feedback process is initiated determines the severity of the brake effect; the entry of nutrients into the last part of the small intestine (the ileum), the ileal brake is a feedback mechanism that results in inhibition of proximal gastrointestinal motility and secretion. The activation of this ileal brake, which is associated with the secretion of gut peptides such as peptide YY (PYY) and GLP-1, results in reduced food intake and increases satiety.
Little is presently known about which nutrients activate the ileal brake. The role of dosage, types and appearances of fat have been established. The role of carbohydrates and proteins remains putative, although, based on recent observations powerful effects of intestinal exposure to these macronutrients can be expected. Therefore we conduct several studies in which we expose the ileum to different amounts of carbohydrates and proteins to see whether carbohydrates or proteins activate the ileal brake. To execute these studies we introduce 200cm long catheter into the small intestine of healthy volunteers. Nutrients can be directly infused through this catheter and can be delivered in different parts of the small intestine.
Information: Dr. Fred Troost, Department of Internal Medicine
Ischemia-reperfusion damage in digestive tract and liver
Ischemia-reperfusion injury is a pathophysiological phenomenon in a variety of diseases. To find new targets for therapy and characterize pathophysiological events during ischemia, innovative human experimental models were developed at the department of Surgery.
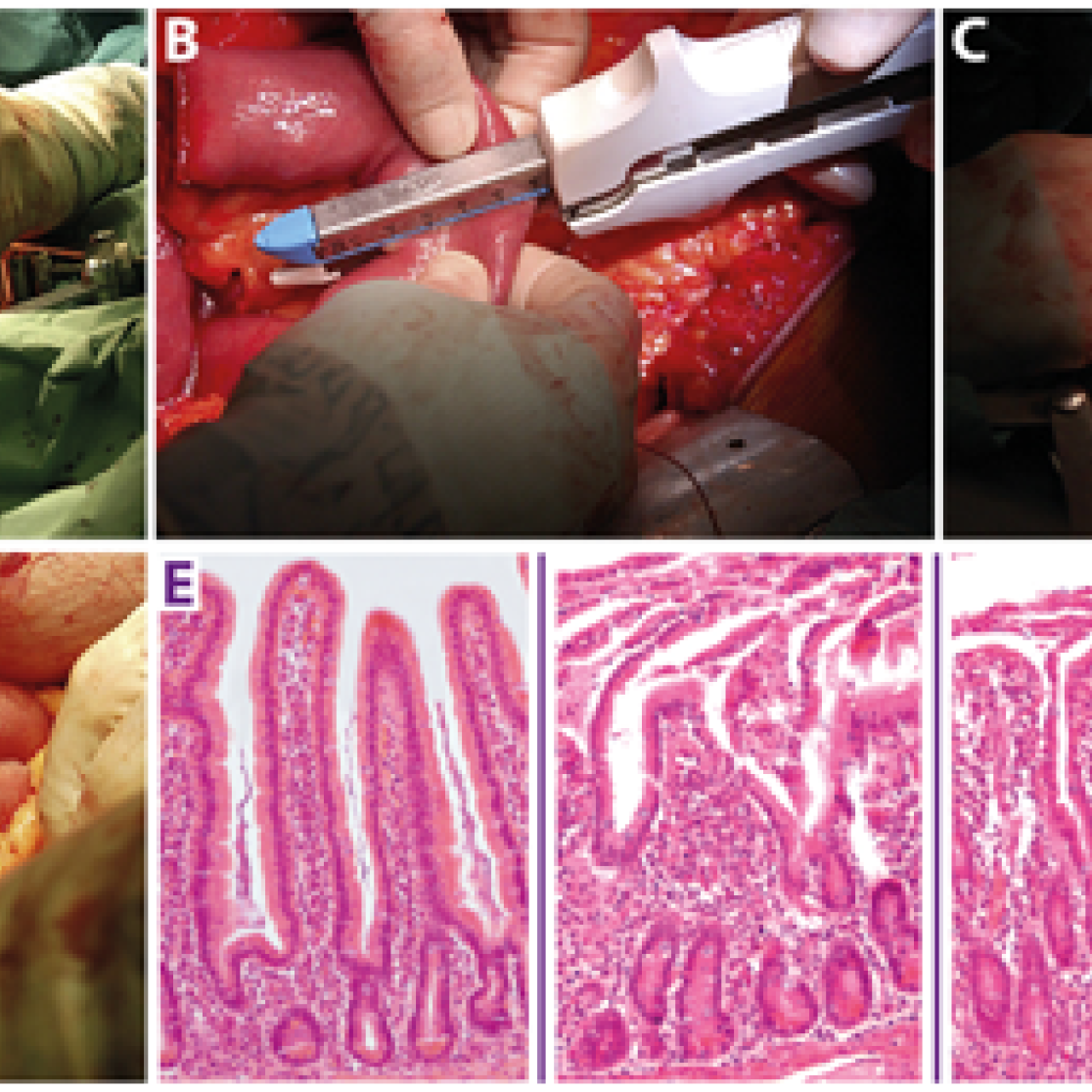
A variable length of healthy jejunum is usually resected in patients undergoing pylorus preserving pancreatico-duodenectomy, of which a small part is used for study purposes. This intestinal segment, provided with a central mesenteric arteriole and venule, (Figure 1A, white arrows indicate mesenteric vessels) is isolated by transection at both ends with a linear cutting stapler (Figure 1B). Thereafter, ischemia is induced for up to 60 min by placing two vascular clamps (Figure 1C, asterisk) across the mesentery. This leads to discoloration of the jejunal wall (Figure 1D, white arrow). Meanwhile, surgery proceeds as planned. Removal of the clamps initiates reperfusion as confirmed by regaining of normal color and restoration of gut motility. Tissue is sampled at different time points throughout the procedure. Blood is sampled at the same time points by direct puncture of the venule draining the isolated jejunal segment. In this manner, we are able to monitor the temporal behavior of biological processes over a period of progressive injury and subsequent initiation of repair. This can be seen from the histological stainings in (Figure 1E) from left to right depicting control tissue, tissue harvested directly after ischemia, and after 30 and 120 minutes of reperfusion. In addition, the blood samples are used for biomarker discovery and to validate our newly developed assays to evaluate intestinal function and integrity. Of note, the procedure only minimally prolongs surgery, making this a harmless, minimally invasive procedure for patients. This model has led to novel insights into human intestinal pathogenesis with respect to barrier disruption, onset of inflammation, cell death, and loss of antimicrobial defense. A similar human model was developed for colon.
Another surgical procedure, the so-called Pringle maneuver during which intermittent hepatic inflow is occluded to minimize blood loss during liver surgery, enables the study of ischemia-reperfusion induced hepatocellular damage.
Information: Prof. Steven Olde Damink, Department of Surgery
Maastricht IBS Cohort: Phenotypical and Genotypical Characterization of Patients with Irritable Bowel Syndrome
Obesity treatment
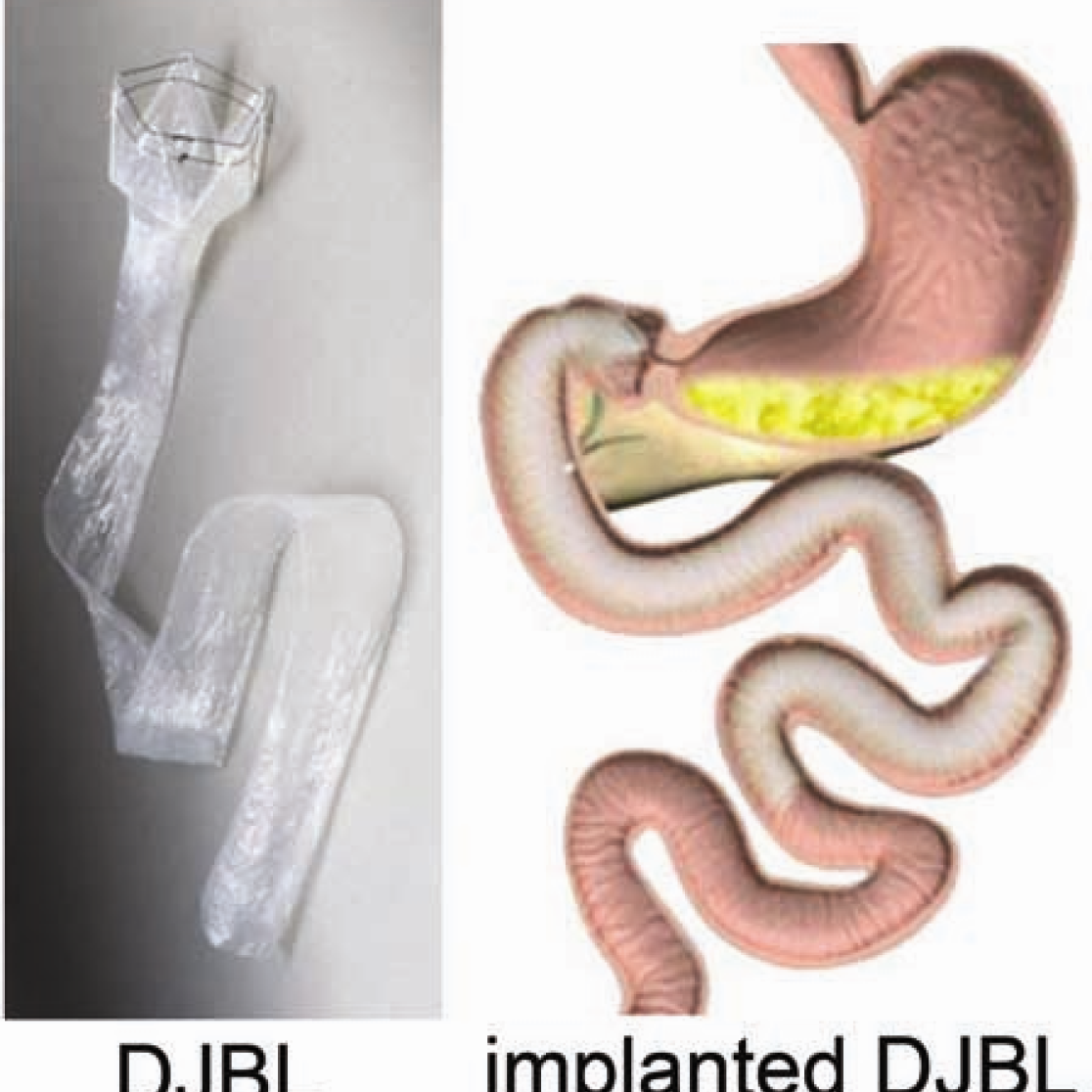
Obesity, type 2 diabetes mellitus, and nonalcoholic fatty liver disease form a worldwide epidemic with great morbidity and mortality. These diseases are treated and studied using innovative surgical techniques. For example, we are pioneering a novel minimally invasive alternative for conventional bariatric surgery, the so-called ‘Endobarrier duodenaljejunal bypass liner’ (DJBL). This device constitutes a 60 cm open teflon sleeve, which is placed endoscopically in the proximal small intestine, effectively excluding this part of the gut from exposure to food. We and others have shown that it rapidly improves type 2 diabetes before significant weight loss occurs. Our most recent data indicate that changes in the postprandial release of the gut hormones GLP-1, GIP, glucagon, and PYY play a major role in the rapid amelioration of type 2 diabetes. Ongoing studies are directed at the DJBL-induced changes in gut microbiota composition, satiety hormones, and plasma parameters of nonalcoholic fatty liver disease.
Information: Prof. Steven Olde Damink, Department of Surgery
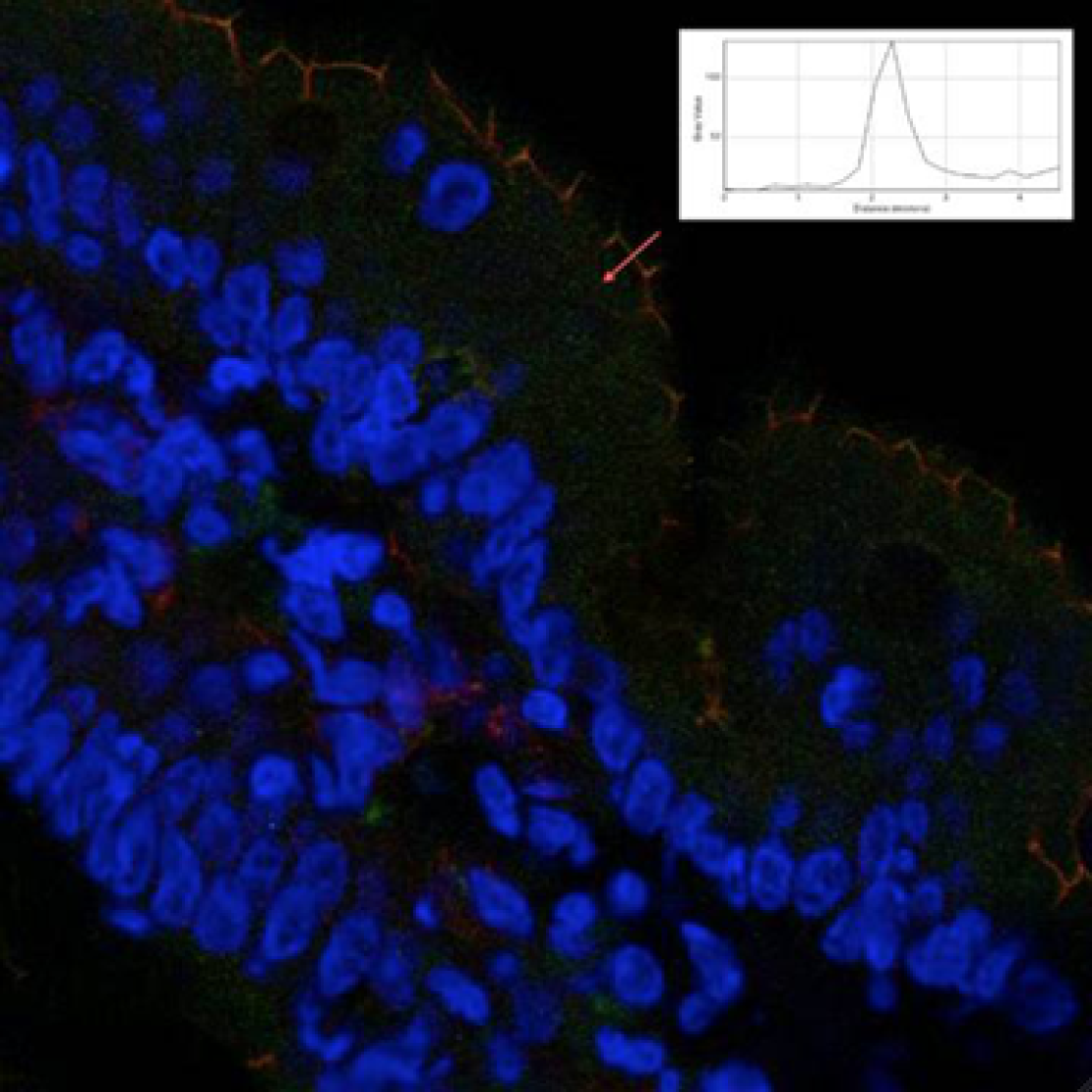
Serotonergic modulation of intestinal permeability and visceral perception
Serotonin (5-hydroxytryptamin, 5-HT) has an important role in regulating human gastrointestinal function.(1-3) 5-HT released from EC cells in response to luminal stimuli acts locally in a paracrine fashion, the major targets being the mucosal projections of sensory afferent neurons. Previous studies suggested the involvement of the 5-HT3 receptors on extrinsic primary afferent neurons in mediating visceral perception.(4) The distribution of the extrinsic sensory innervation in close proximity to enterochromaffin cells as the sources of 5-HT, in conjunction with their demonstrated sensitivity to this mediator, point to a role for 5-HT in the transduction of visceral stimuli.
Besides a role in visceral perception, a number of studies in vitro have shown that 5-HT affects tight junctions in Caco-2 intestinal epithelial (5) and can therefore regulate intestinal barrier function. Disturbances on intestinal barrier function have been associated with irritable bowel syndrome, along with alterations in visceral perception and serotonergic metabolism.
In a study using the precursor of 5-HT, 5-hydroxytryptophan, we demonstrated that oral intake of 5-HTP resulted in significant changes in mucosal and systemic 5-HT metabolites. We also observed an increase in visceroperception of pain in both healthy volunteers and IBS patients, which was determined by rectal mechanical distensions (barostat). Furthermore, we assessed intestinal barrier function by taking biopsies from the small intestine and administering a multi-sugar drink and measuring plasma recovery of the sugars. In healthy controls, a 5-HTP induced reinforcement of the intestinal barrier function was seen, while the opposite reaction was observed in IBS patients. This could indicate the presence of a serotonin-mediated mechanism aimed to reinforce intestinal barrier function which appears to malfunction in IBS patients, potentially contributing to disease development.
Information: Dr. Fred Troost, Department of Internal Medicine