The application of intrinsically labeled milk protein in human nutrition research
Division 3: Respiratory & Age-related Health
Department of Human Biology
Background
Food ingestion plays an important role in maintaining muscle mass and strength. Ingestion of protein provides us with amino acids that we require as building blocks for our own muscle tissue. However, amino acids can also act as signaling molecules, directly activating molecular pathways that stimulate muscle growth and repair. The capacity of a dietary protein to stimulate protein synthesis largely depends on its protein digestion and amino acid absorption kinetics. These processes have proven difficult to study in an in vivo human setting. To study the process of protein digestion, amino acid absorption, and the subsequent incorporation in skeletal muscle tissue we developed a novel method for which we produced intrinsically, stable isotope labeled milk protein. By infusing a large amount of stable isotope labeled amino acids in a lactating cow, collecting its milk, and extracting the protein we managed to produce a protein source that we could follow throughout the human body, from ingestion all the way to its use for human tissue protein synthesis (Figure 1).
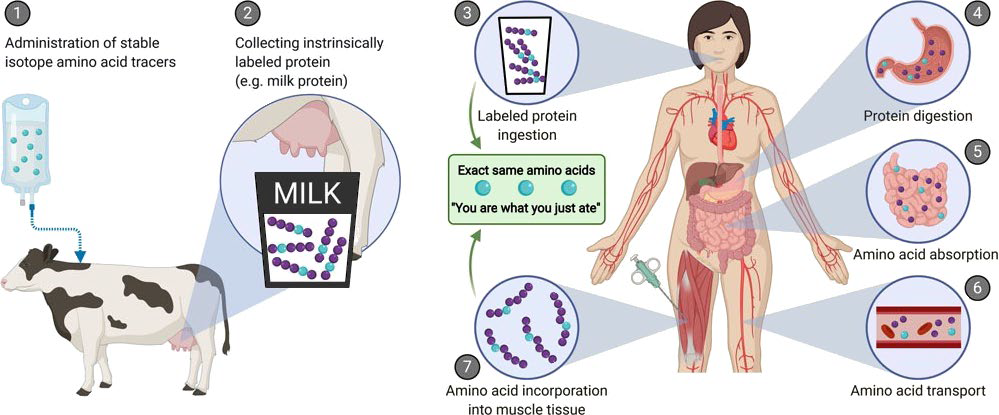
Figure 1: Schematic representation of the production of intrinsically labelled protein to assess various aspects of post-prandial protein handling. Here, the production of intrinsically labelled milk: (1) stable isotope amino acid tracers are administered to lactating cows, (2) the cow produces milk with the amino acid tracer incorporated into the milk protein matrix. Application of intrinsically labelled protein: (3) the collected intrinsically labelled milk protein is consumed by participants, (4) dietary protein is digested into amino acids, (5) dietary protein-derived amino acids are taken up in the gastrointestinal tract, (6) dietary protein-derived amino acids are released into the circulation and (7) dietary protein-derived amino acids are taken up and incorporated into tissues, such as skeletal muscle.
Major breakthroughs
The application of intrinsically labelled protein has revealed that dietary protein-derived plasma amino acid availability can be strongly modulated by numerous nutritional and non-nutritional factors. This is of important clinical relevance, since dietary protein-derived amino acid availability is the main determinant driving the post-prandial increase in skeletal muscle protein synthesis rates. Intrinsically labelled protein is now frequently being applied to investigate how muscle protein synthesis rates are modulated by the various aspects of post-prandial protein handling. It has contributed largely to our understanding how nutrition and physical (in) activity interact in determining muscle quality in both health and disease (Figure 2).
The use of intrinsically labelled proteins will facilitate our efforts to understand the impact various nutritional and non-nutritional factors have on post-prandial protein handling and, as such, how they can modulate muscle quality in both health and disease.
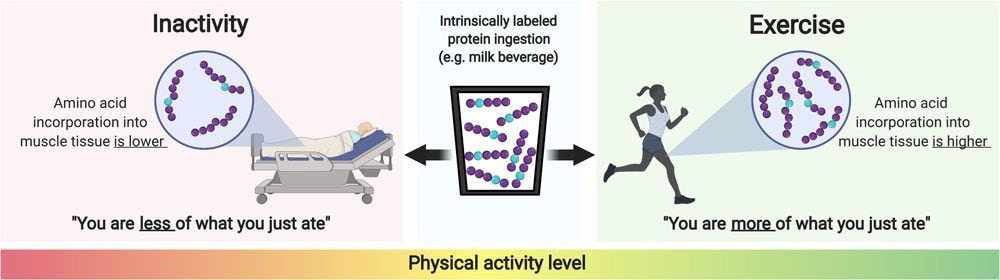
Figure 2: Schematic representation of the impact of physical (in)activity on the incorporation of dietary protein-derived amino acids into skeletal muscle protein.
Users and collaborations
The work leading up to the development of the method, the application in human nutrition research, and the subsequent use of these insights in education, healthcare and product research and development represents a team effort including both academic, medical, and industry partners.
Academic and medical collaborators include: Universite ́ Clermont Auvergne, Wageningen University, INRA, University of Exeter, Karolinska Institutet, Royal Adelaide Hospital, Australian Catholic University, University of Birmingham, Virga Jessa Hospital and the University Medical School in Nottingham. Industrial partners include DSM, Friesland Campina, Danone/Nutricia, Kellogg, Syral, Cargill, Pepsico, and many more. Top Institute Food and Nutrition and the Dutch TKI have been instrumental in bringing all parties together. The list of partners will continue to grow as we strive for even more fruitful collaborations.
Scientific impact/Research quality
The scientific output based upon the described method and its application has been extensive, with more than 50 high-impact, peer reviewed publications (a selection is provided below) and at least 10 successful PhD theses. Besides the publication record, the method and the insights it has provided have been presented at numerous conferences and received various Young Investigator Awards in different fields of research.
Selection of publications
1. Gorissen SHM, Trommelen J, Kouw IWK, Holwerda AM, Pennings B, Groen BBL, Wall BT, Churchward-Venne TA, Horstman AMH, Koopman R, Burd NA, Fuchs CJ, Dirks ML, Res PT, Senden JMG, Steijns J, de Groot L, Verdijk LB, van Loon LJC. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J Nutr. 2020. www.ncbi.nlm.nih.gov/pubmed/32069356 [IF:4.423] [Altmetric score: 43].
2. Horstman AMH, Kouw IWK, van Dijk JW, Hamer HM, Groen BBL, van Kranenburg J, Gorissen SHM, van Loon LJC.
The muscle protein synthetic response to whey protein ingestion is greater in middle-aged women when compared with men. J Clin Endocrinol Metab.
2019;104(4):994–1004. www.ncbi.nlm.nih.gov/pubmed/30423113 [IF:6.215] [Altmetric score: 41].
3. Trommelen J, Kouw IWK, Holwerda AM, Snijders T, Halson SL, Rollo I, Verdijk LB, van Loon LJC. Presleep dietary protein-derived amino acids are incorporated in myofibrillar4- protein during post-exercise overnight recovery. Am J Physiol Endocrinol Metab. 2018;314(5):E457-E67. www.ncbi.nlm.nih.gov/pubmed/28536184 [IF:4.248] [Altmetric score: 136].
4. Gorissen SH, Horstman AM, Franssen R, Kouw IW, Wall BT, Burd NA, de Groot LC, van Loon LJC. Habituation to low or high protein intake does not modulate basal or postprandial muscle protein synthesis rates: a randomized trial. Am J Clin Nutr. 2017;105(2):332-42. www.ncbi.nlm. nih.gov/pubmed/27903518 [IF:7.506] [Altmetric score: 17].
5. Wall BT, Dirks ML, Snijders T, van Dijk JW, Fritsch M, Verdijk LB, van Loon LJC. Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion. Am J Physiol Endocrinol Metab. 2016;310(2):E137-47.www.ncbi.nlm.nih.gov/pubmed/26578714 [IF:4.248] [Altmetric score: 38].
6. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJC. Aging Is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One. 2015;10(11): e0140903. www.ncbi.nlm.nih.gov/pubmed/26536130 [IF:3.394] [Altmetric score: 22].
7. Gorissen SH, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJC. Carbohydrate co-ingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab. 2014;99(6):2250-8. www.ncbi.nlm.nih.gov/pubmed/24628553 [IF:6.215] [Altmetric score: 28].
Wall BT, Snijders T, Senden JM, Ottenbros CL, Gijsen AP, Verdijk LB, van Loon LJC. Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J Clin Endocrinol Metab. 2013;98(12): 4872-81. www.ncbi.nlm. nih.gov/pubmed/24108315 [IF:6.215] [Altmetric score: 14].
9. Groen BB, Res PT, Pennings B, Hertle E, Senden JM, Saris WH, van Loon LJC. Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am J Physiol Endocrinol Metab. 2012;302(1):E52-60. www.ncbi.nlm.nih.gov/pubmed/21917635 [IF:4.248] [Altmetric score: 78].
10. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJC. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93(2):322-31.www.ncbi.nlm.nih. gov/pubmed/21084649 [IF:7.506] [Altmetric score: 30].
Who is involved?
The M3-research unit is part of the Department of Human Biology and includes 4 expert technicians, 3 post-doctoral fellows, and more than 12 PhD students, supervised by Dr. Tim Snijders (assistant professor), Dr. Lex Verdijk (associate professor) and Dr. Luc van Loon (professor). The research group specialises in in vivo human metabolic research, with skeletal muscle metabolism, exercise metabolism, sports and clinical nutrition, and aging as the main fields of interest. The group has been successful in the acquisition of more that 25 M€ of research funding, mainly through building large public-private partnerships. The stable isotope analytical facilities at Maastricht University Medical Centre are provided by the Stable Isotope Research Centre (SIRC).
Societal impact
The applied method and its application have provided us with a more comprehensive insight in the digestion and absorption of protein from our diet and its subsequent impact on muscle protein synthesis. Moreover, it has provided us with a more holistic view on how physical activity and nutrition can modulate health. Such a comprehensive assessment of the various processes involved in post-prandial protein handling facilitates the transfer of our research output towards the general public. Besides many interviews, podcasts and lectures, several popular scientific instruction videos have been made by the University of the Netherlands to educate the general public on the fact that we can actually show that ‘you are what you eat’.
www.youtube.com/watch?v=huTlXfKHtVQ
www.youtube.com/watch?v=mCRzi1tObe0
Future perspectives
This novel approach will further extend the use of stable isotope tracers in nutrition research as other research groups are now applying the method extensively and its application will continue to grow among researchers in the field. Further innovations include the recent successful production and application of intrinsically labeled insects. There is a growing interest in insects as an alternative source of dietary protein for human consumption that may be produced on a more viable and sustainable commercial scale and, as such, will contribute to ensuring global food security. We have successfully produced intrinsically labeled insects allowing us to evaluate the bioavailability and functional properties of this protein source following their consumption in vivo in humans.