Heart Failure
Heart failure research within the department of Physiology focuses on the role of abnormal electrical conduction as a cause of atypical contraction and subsequent development of heart failure. In addition, the potential to prevent or treat heart failure by improved cardiac electrical activation is investigated.
Experimental approaches:
- Isolated cardiomyocytes and cardiac fibroblasts in 2D (figure 1) and 3D (co)-culture (figure 2). These cellular studies enable the investigation of the effect electromechanical stimuli on cell function and interaction.
- Large animal studies (pigs) using models of dyssynchrony (ventricular pacing, left bundle branch block) and myocardial infarction. These experiments allow extensive and detailed measurements of hemodynamics and electrical conduction as well as long term effects of pacemakers therapies.
- Clinical studies in patients with pacemakers, implanted for treatment of bradycardia or heart failure. Small proof-of-principle studies have been performed, using ideas stemming from results of aforementioned animal experiments and computer simulations.
- Fit experimental and clinical data with computer simulations using the Circadapt model: close collaboration with the department of Biomedical Engineering. This provides unique ways to study the effects of abnormal cardiac activation in conjunction with hemodynamic variabilities.
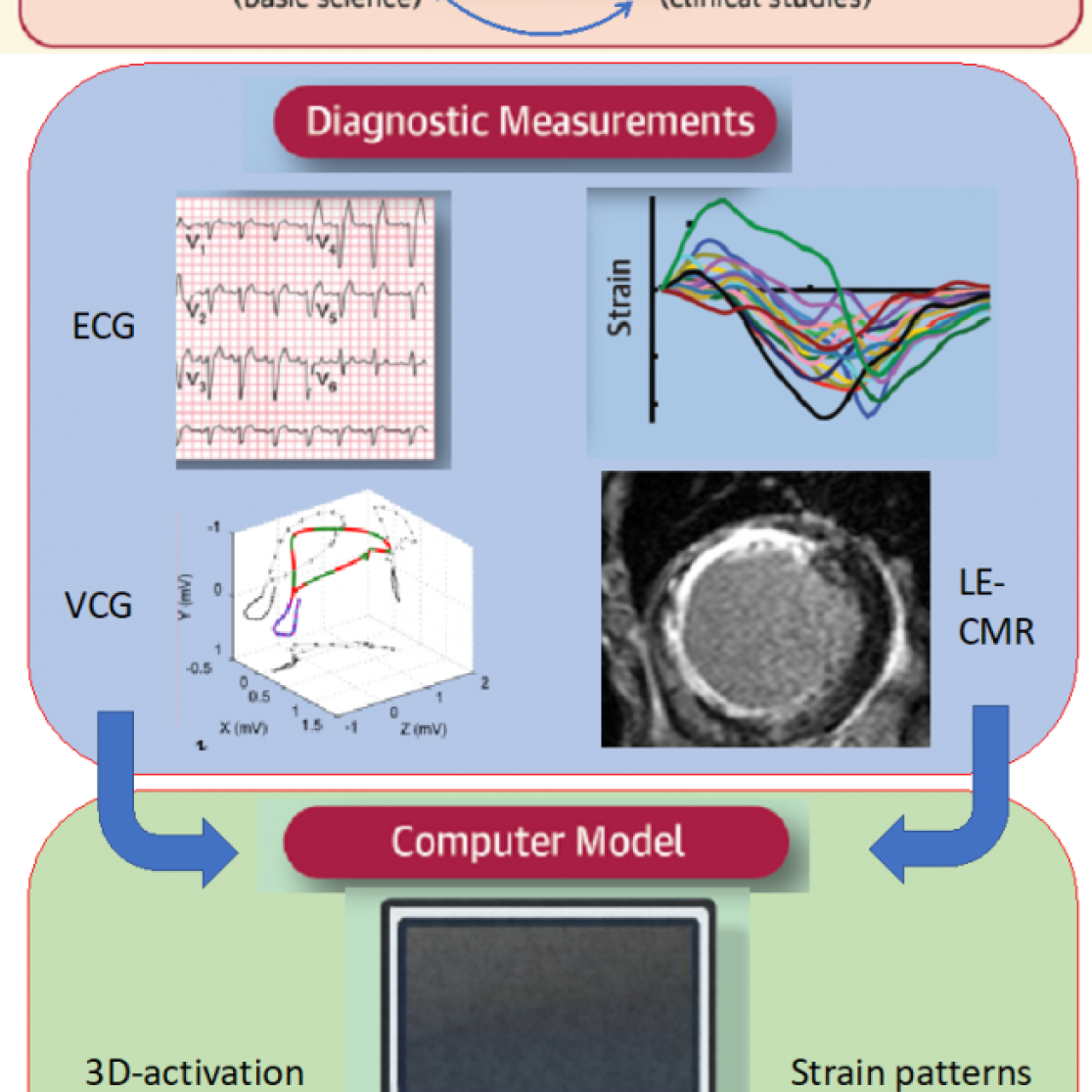
The use of all approaches mentioned above for improving cardiac resynchronization is illustrated by figure 5
Main research questions:
- Finding better site(s) for pacing in patients with pacing indications because of bradycardia or heart failure, such as the endocardium of the left ventricle, left side of the septum and the area near the left bundle branch.
- Novel, smart tools to optimize the time interval between stimulation of the atrium and the right and left ventricle.
- Investigate adaptations at the (sub)cellular and molecular level in dyssynchronous and resynchronized hearts.
- Search biomarkers that better predict the success of cardiac resynchronization therapy, in particular electrical (ECG) and mechanical markers (echocardiography, MRI).
Figure 1. The Flexcell system can cyclically stretch isolated cells for several days.
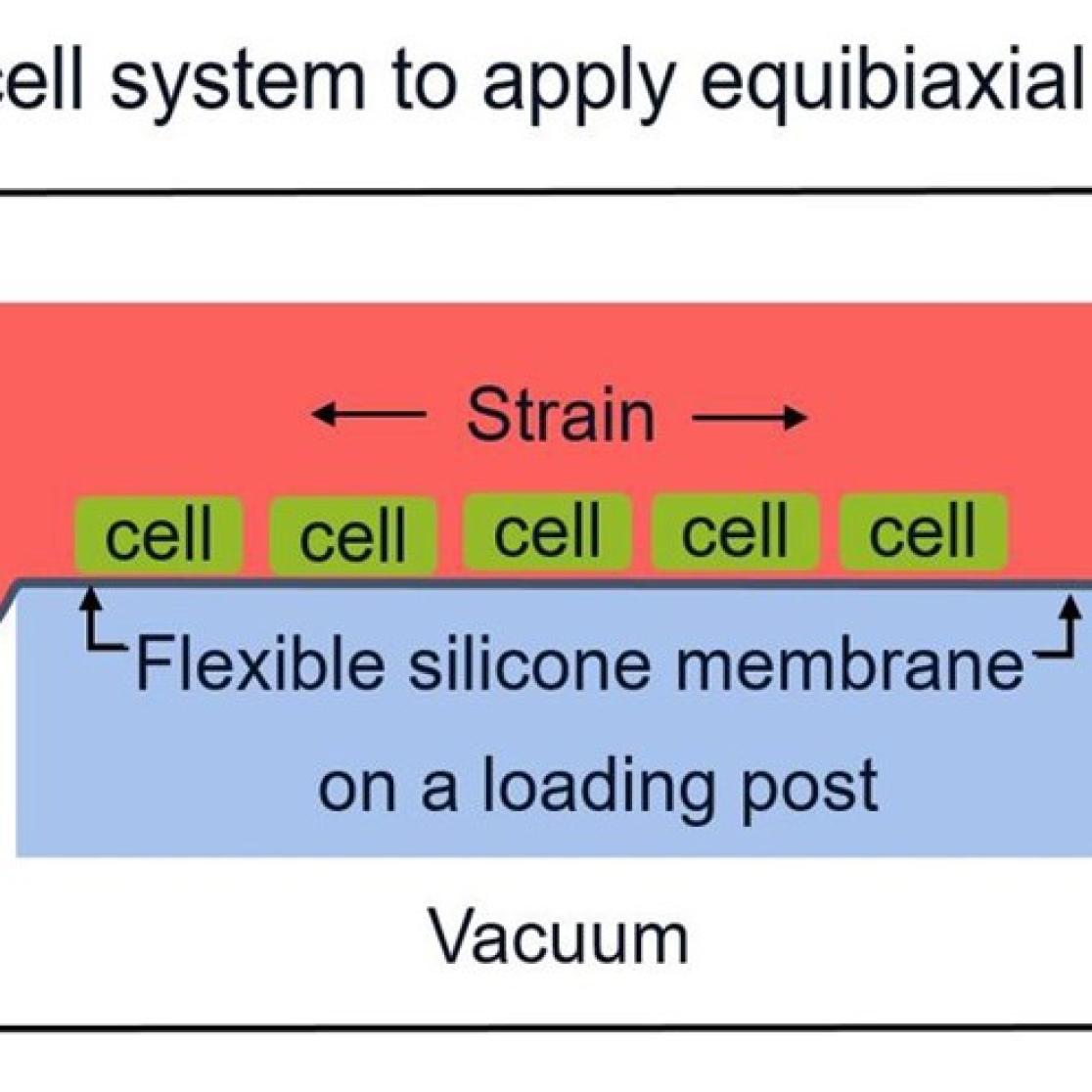
Figure 2: To create a 3D ring of engineered heart matrix, cardiac fibroblasts are cultured in a collagen hydrogel in a silicone mold (upper left), resulting in a ring structure (upper right) that can be stretched subsequently (lower).
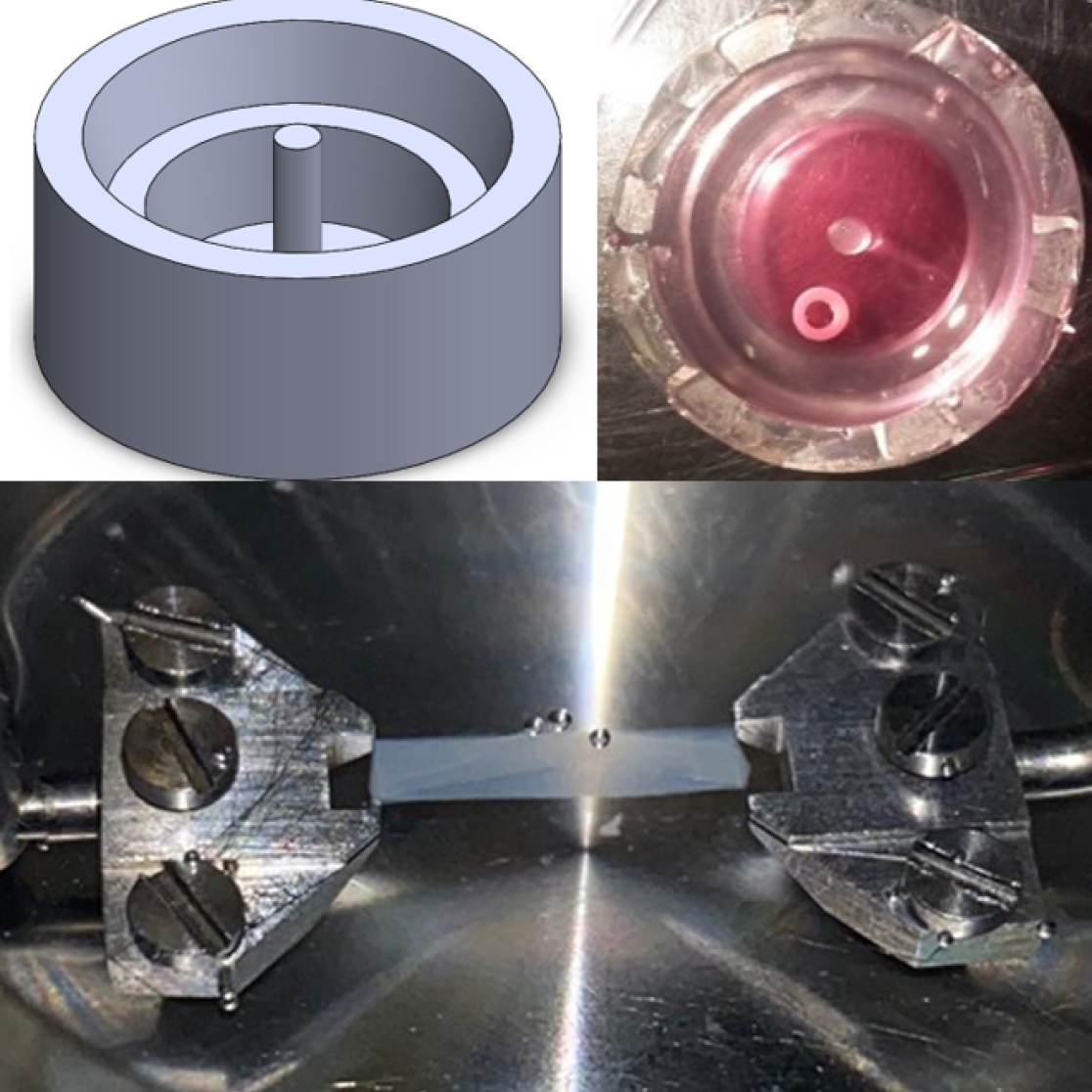
Figure 3. example of matching of animal experiments with the Circadapt computer program. Electrical activation, measured by electrodes on the epicardium (signals upper left) and pump function (upper right) were measured in animal experiments. Electrical activation data were used as input for computer simulations that proved to replicate pump function (validation). Following validation, additional information on heart and circulation were predicted by the model.
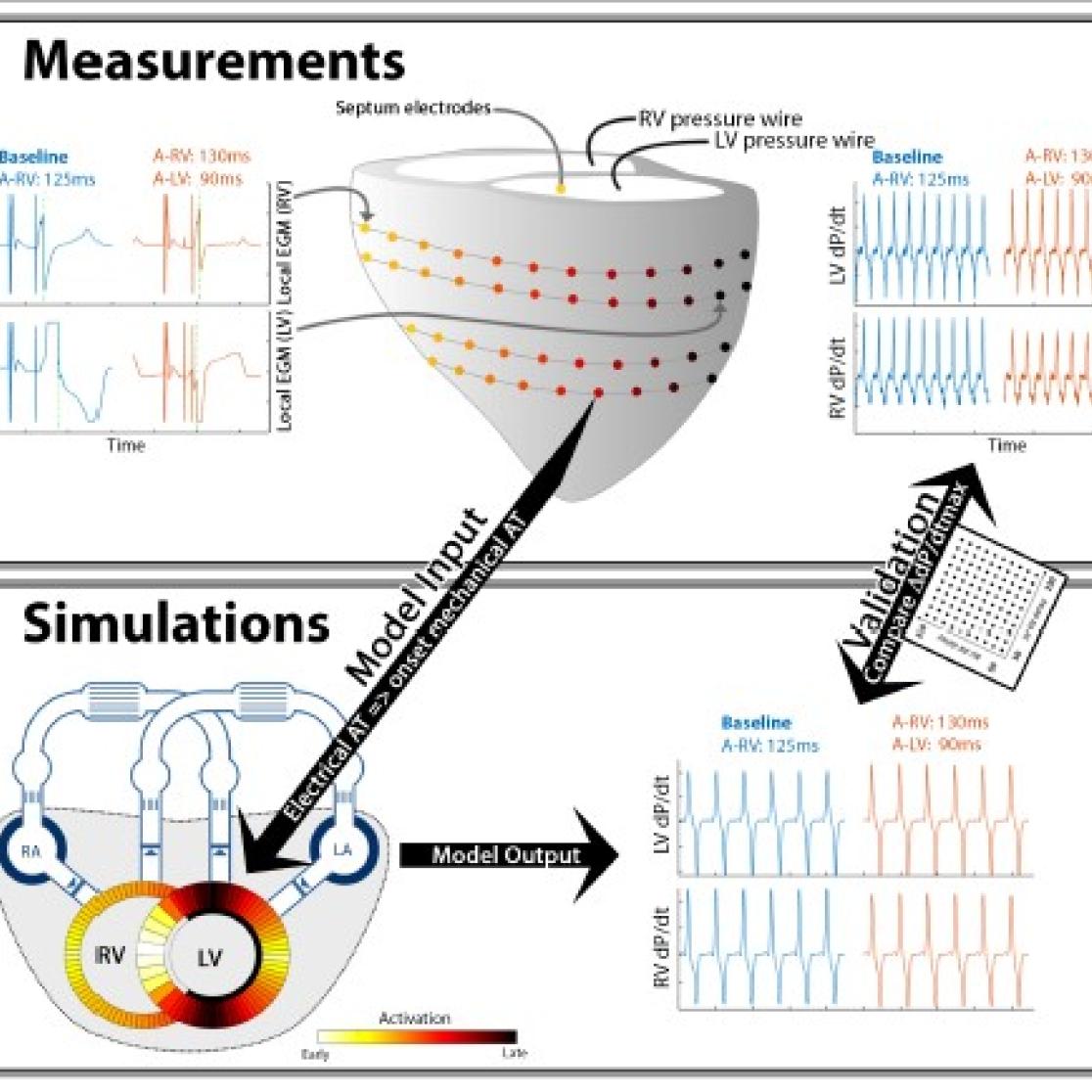
Figure 4. Example of clinical study. Animal experiments had shown good performance of left ventricular (LV) septal pacing in case of conduction abnormalities. LV septaum pacing was compared with conventional biventricular pacing in patients using body surface mapping and LV pressure measurements. LV septum pacing performed at least as good as biventricular pacing, while it requires one electrode less to be implanted.
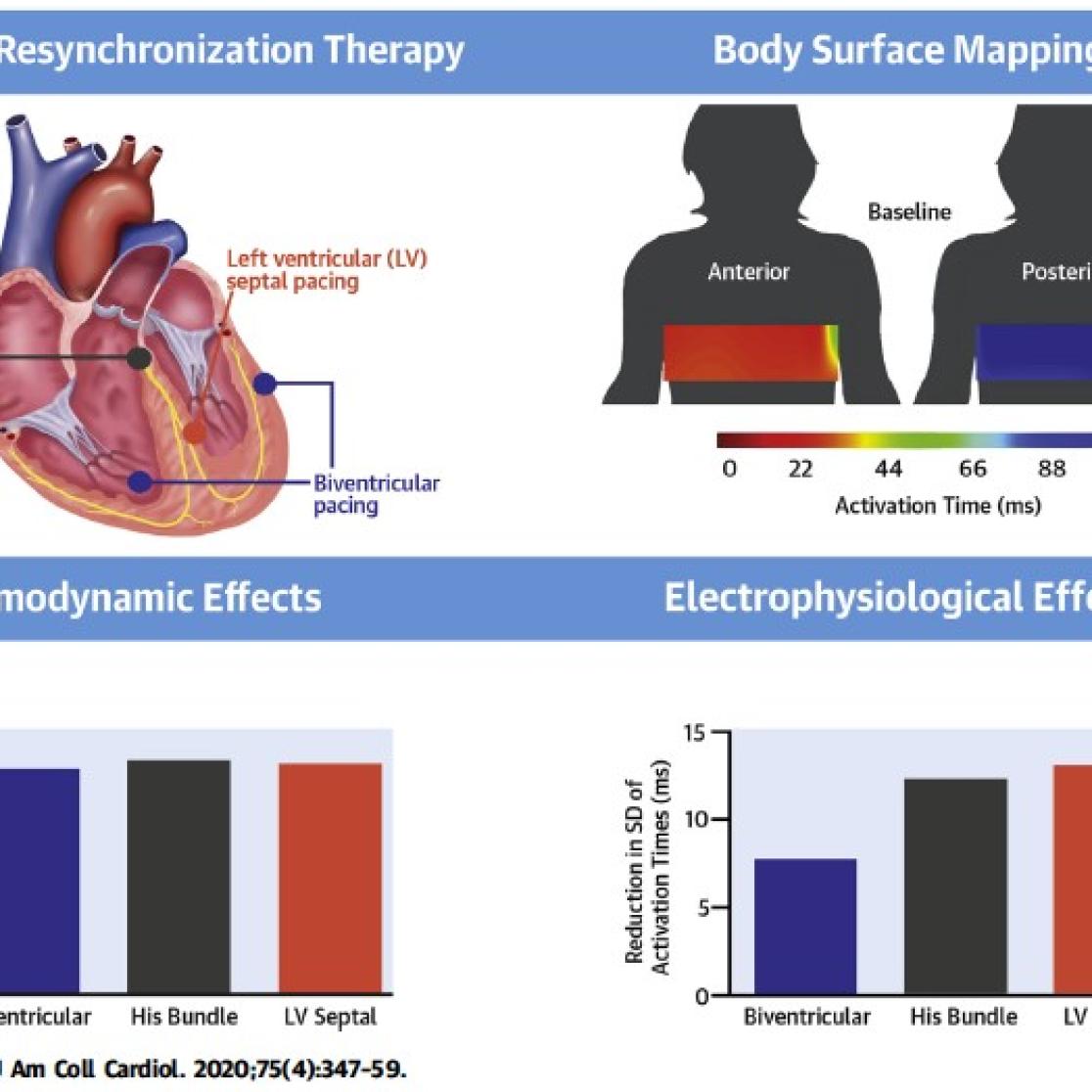
Figure 5. integral few how the various experimental approaches can be combined