Atrial Fibrillation
Click here for a recent publication list of U. Schotten
Cardiac Electrophysiology Group
The Cardiac Electrophysiology Group within the Department of Physiology has a long track record for research into the pathophysiological mechanisms of atrial fibrillation. Research within the Electrophysiology groups spans the translational axis from biochemistry and subcellular compartmentation to implementation of treatment in AF patients.

Cellular proarrythmic mechanisms
Although calcium handling has been investigated extensively in ventricular myocytes, far less is known about celllular calcium homeostasis in the atrium. We are studying cellular proarrhythmic mechanisms in isolated atrial myocytes from several experimental models and AF patients. We are also investigating nuclear calcium signalling in atrial myocytes and its effect on gene expression (excitation-transcription coupling). The contribution of cellular proarrhythmic event to the maintenance of AF is further investigated in in vivo experiments (see below).
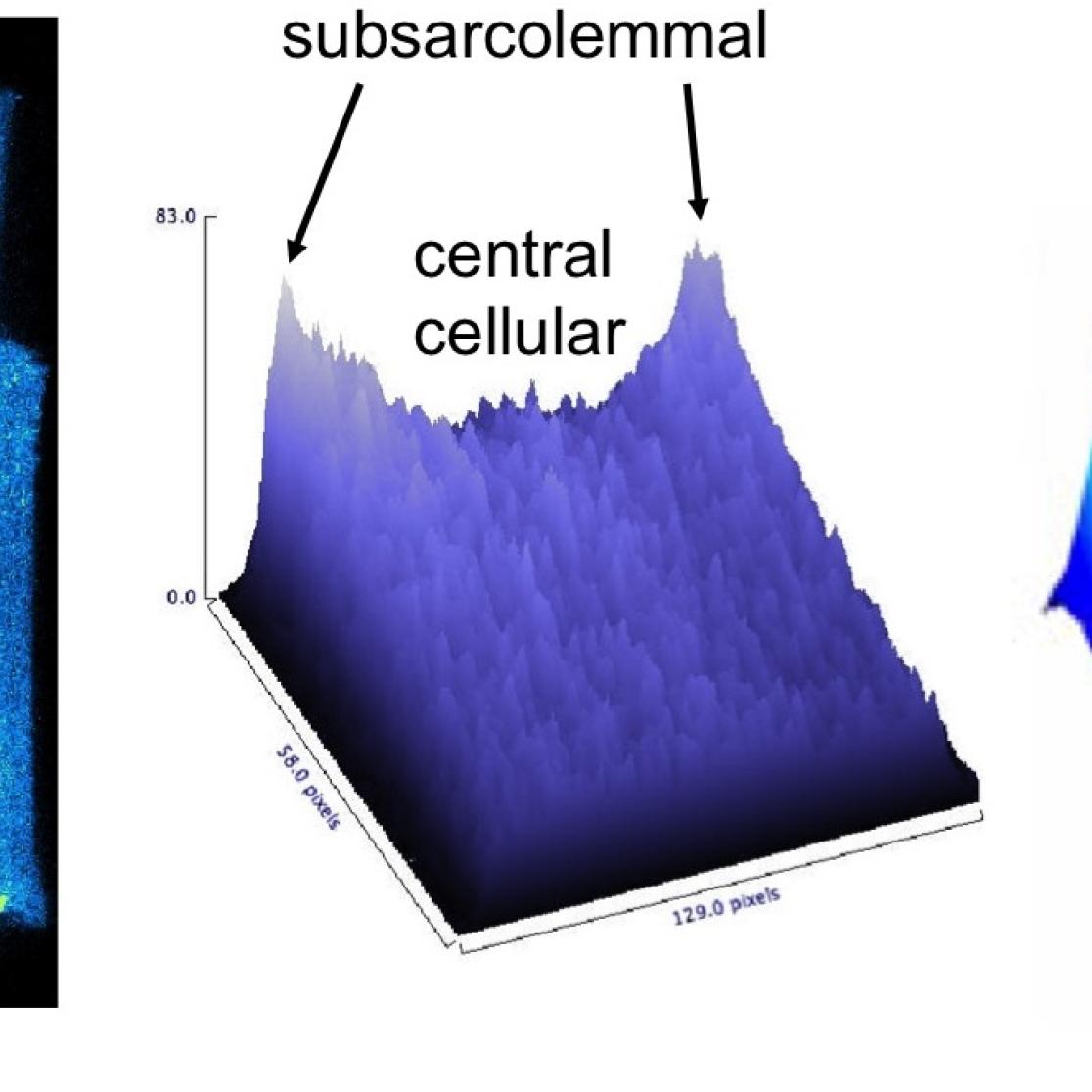
Left group of panels: calcium sparks leading to a calcium waves in a single myocyte.
Middle panel: within the myocyte, the calcium transient is fast and large in the subsarcolemmal space, but it decreases in amplitude when it spreads towards the center of the cell (atrial myocytes lack a T-tubular system). Right panel: the origin of the nuclear calcium transient is still matter of debate, and we are investigating whether it can be completely explained by simple diffusion of the cytoplasmic calcium transient or whether there is a significant contribution of intrinsic nuclear calcium release mechanisms.
AF complexity and structure-function relation of the atrium
AF leads to several alterations in atrial cellular electrophysiology (electrical remodeling) and atrial tissue structure (structural remodeling). We are studying the relation between atrial remodeling (histology and MRI) and atrial conduction (direct contact mapping and optical mapping)
Left panel: the atrial anatomy is complex, with an intricate network of endocardial trabeculae underlying a thin epicardial layer. Right panel: long term AF leads to endomysial fibrosis in the epicardial layer (upper panels), which is associated with electrical uncoupling of myocyte strands in the transverse direction (lower panels). During AF these structural alterations leads to a more 3-dimensional and more stable fibrillation pattern. For more details, Verheule et al Circ A&E 2013;6:202-11, Eckstein et al Circ A&E. 2013;6:334-41).
The slow process of structural remodeling and the resulting disruption of side-to-side connections between atrial muscle bundles leads to a gradual increase in the complexity of fibrillation patterns and in the stability of AF itself. To investigate fibrillatory conduction and characterized the AF substrate, we have developed several sophisticated mapping and analysis tools.
Fibrillation patterns in persistent AF (left panel) are much simpler than those in permanent AF (second panel). To investigate the 3-dimensional nature of these patterns, a novel, clamp-like mapping tool was developed (middle panel). This tool enable us to study in vivo whether fibrillation waves are generated by transmural conduction (second column from right) or by focal activity (right column), For more details, read Verheule et al Circ A&E. 2010;3:590-9, Verheule et al Circ A&E 2013;6:202-11, Eckstein et al Circ A&E. 2013;6:334-41.
Automated fibrillation pattern analysis and substrate stratification in AF patients
Detailed information on the AF substrate would provide an extremely valuable tool to guide treatment strategies such as radio frequency ablation. However, the time-and labor-intensive nature of mapping of fibrillation patterns has severely limited its clinical applicability. For this reason, we have developed fully validated methods for fast, automated analysis.
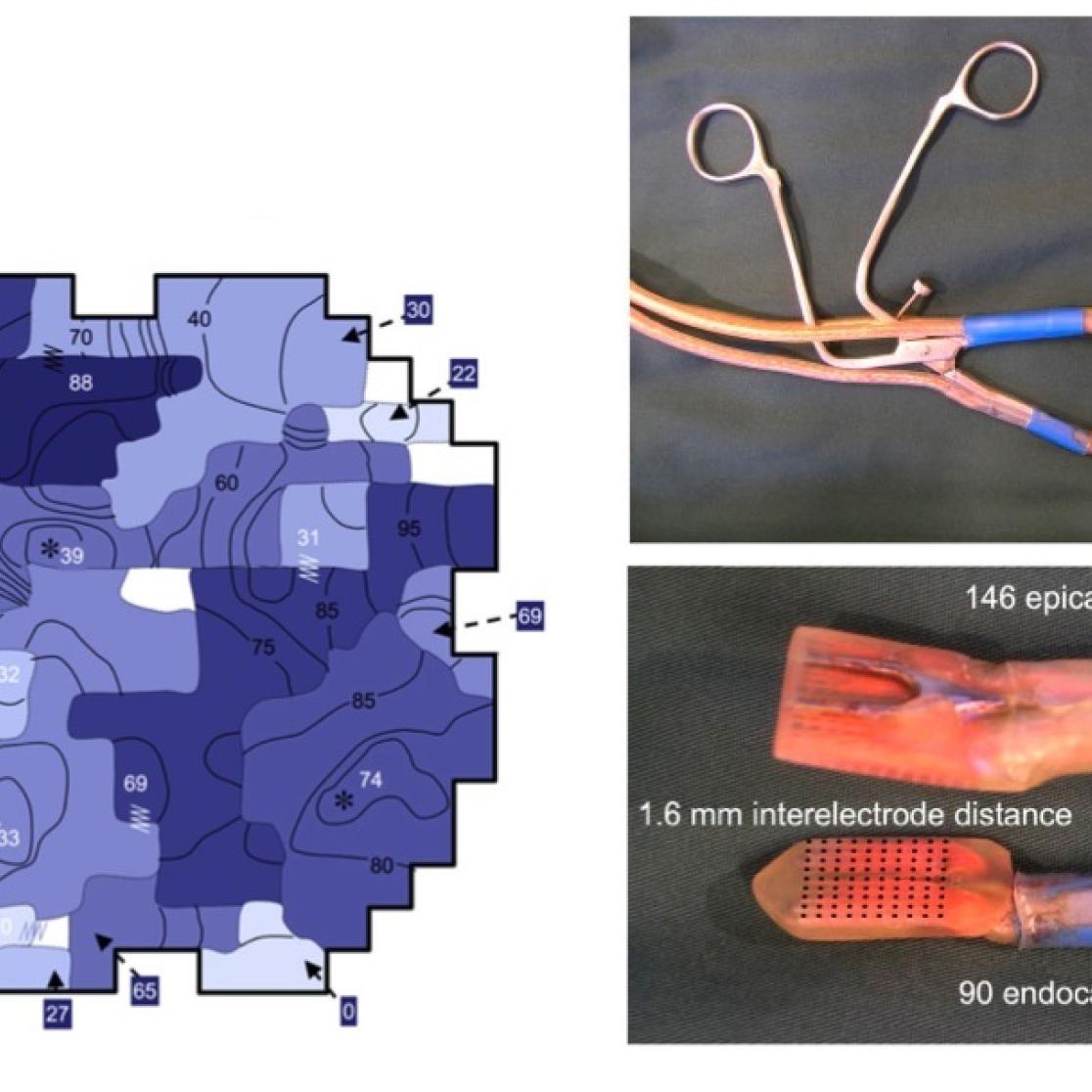
Left panel: novel computer algorithms for automated analysis of individual fibrillation electrograms (template matching and probabilistic activation detection). Right panel: the derived activation times can subsequently be used in automated reconstruction of conduction patterns during AF. The examples illustrate the difference in degree of AF complexity in patients with paroxysmal and longstanding persistent AF. For more details, see Zeemering S, et al. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:6357-60
Atrial vascular function
Like the ventricular myocardium, the atria have a dense capillary bed. We are investigating the regulation of the atrial blood supply and whether this supply is sufficient under conditions of increased metabolic demand (e.g. acute AF).
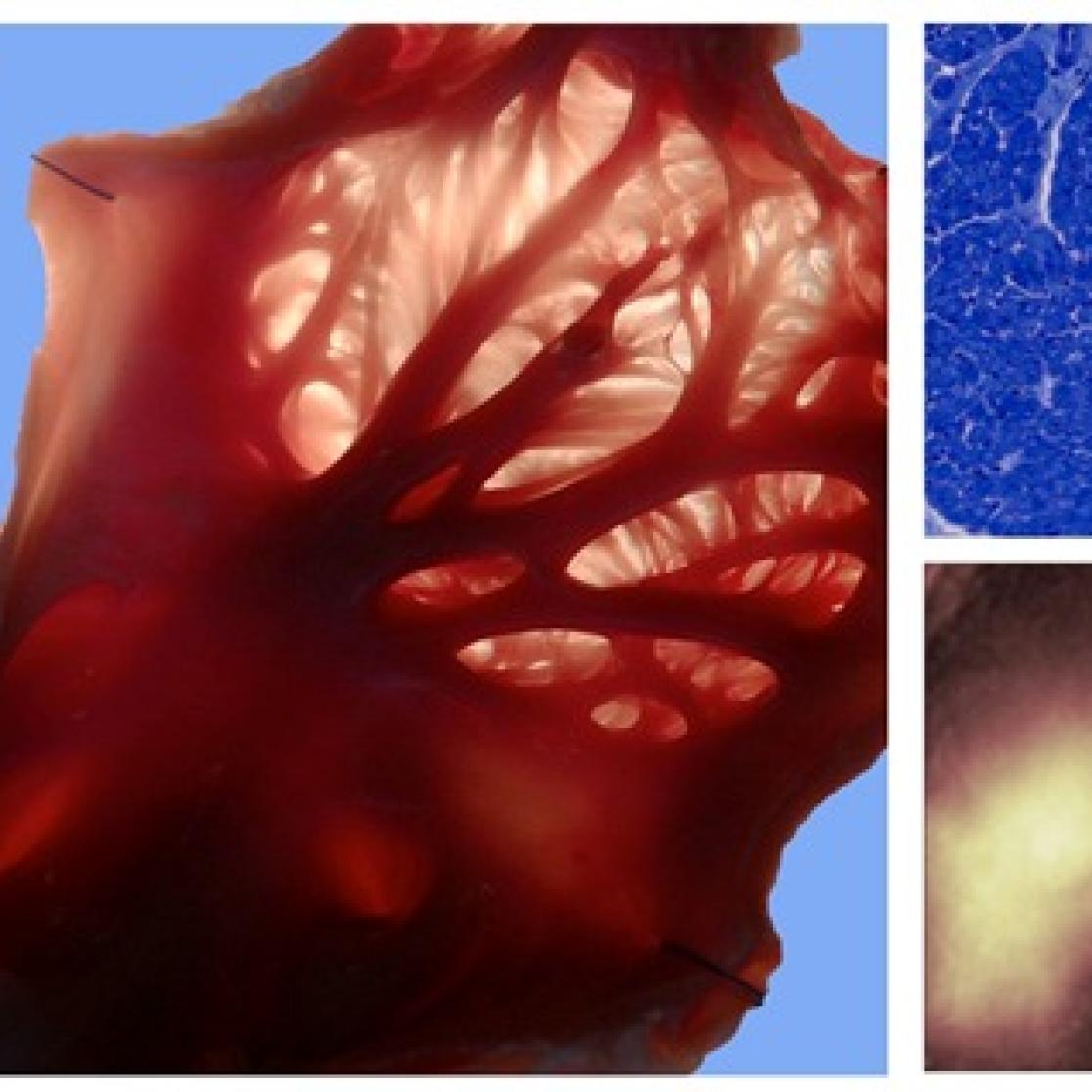
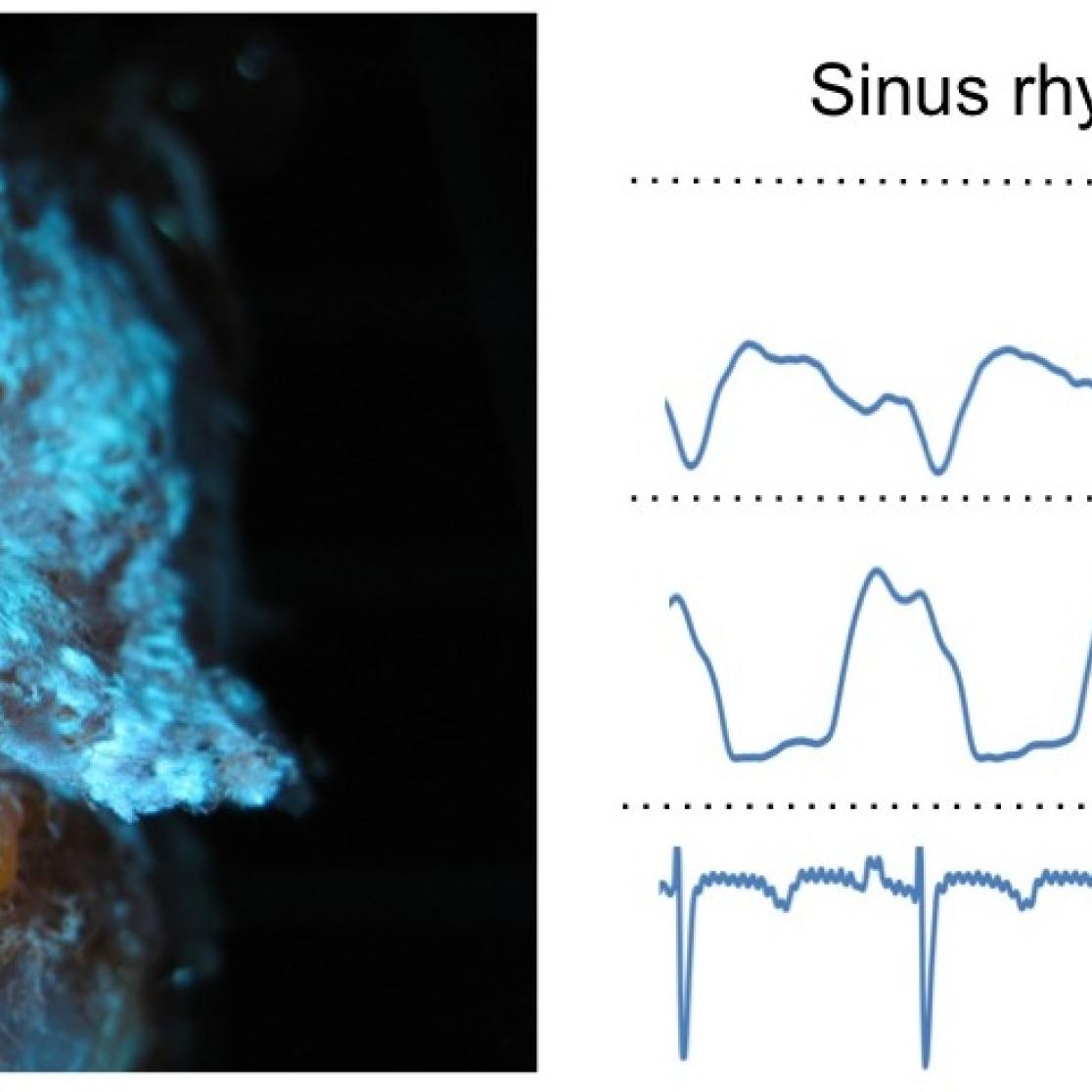
Left panel: vascular corrosion cast showing the circumflex artery and the left atrial and ventricular vascular beds. Right panels: coronary flow measurement in left atrial and ventricular arterial branches during sinus rhythm and AF.
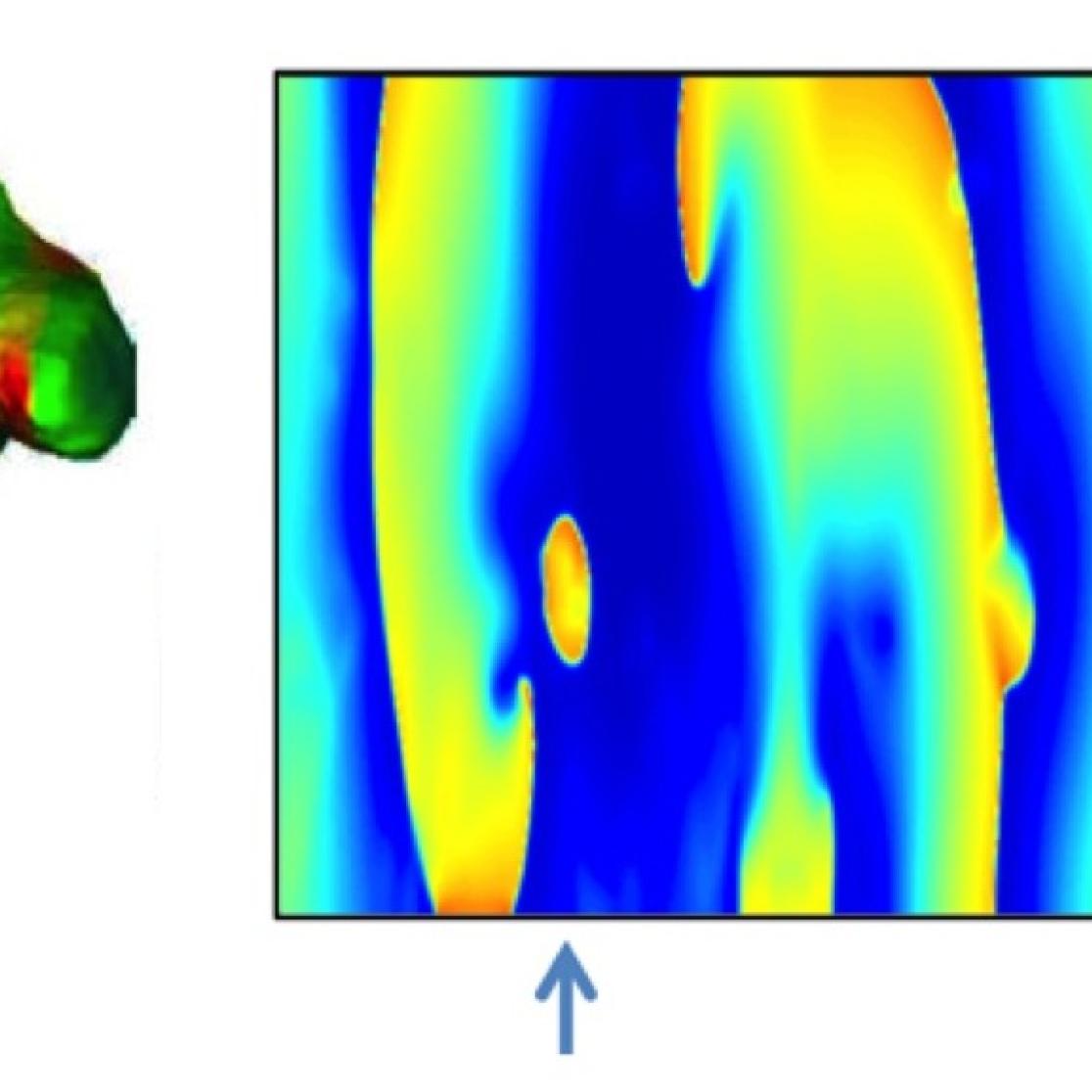
Left panel: fibrillation pattern in a 3-dimensional representation of the atrial anatomy. Right panels: epi- and endocardial maps in a simple proof-of-principle model of transmural conduction that is used to study the behavior of epicardial breakthrough waves (for more details, read Kuipers et al, Heart Rhythm. 2011;8:429-36, Gharaviri et al Europace. 2012;14 Suppl 5:v10-v16, Verheule et al Circ A&E. 2013;6:202-11).
Research facilities
Depicted below are some examples of our experimental setups (clockwise from top left): electrophysiological and hemodynamic measurement during sterile surgery in our operating room; high-density simultaneous mapping epi- and endocardial conduction patterns; mapping of fibrillatory conduction patterns in an AF patient during coronary bypass surgery; high-resolution optical mapping for perfused preparations; combined patch clamp electrophysiology and confocal microscopy.
