Impaired organ blood flow in obesity and diabetes
Principal Investigator: Etto Eringa
Description of research
Introduction
Our research focuses on impaired microvascular functions in obesity and type 2 diabetes, which contribute to organ failure in these conditions. The hallmark of which is impairment of organ blood flow, which occurs early in the pathogenesis of type diabetes and is detectable before symptoms of disease. Microvascular dysfunction and impaired organ blood flow result from complex interactions between fat tissue, the kidneys and the immune system.
Microvascular dysfunction in obesity, diabetes and organ failure
The microcirculation of different organs has different functions, including regulation of local blood flow, inflammation and metabolism. In turn these microvascular functions control organ function and dysfunction, including heart failure, myocardial ischemia and cognitive decline. Diabetes, hypertension and obesity, known as the metabolic syndrome in combination with dyslipidemia, are chronic conditions associated with impaired organ blood flow and inflammation. Microvascular dysfunction in the metabolic syndrome is characterized by increased arteriolar resistance, reduced capillary density and blood flow and an imbalance between endothelial secretion of vasodilator and vasoconstrictor substances. A type of microvascular dysfunction specific to the metabolic syndrome is impairment of insulin-induced vasodilation. While well-known in organ physiology and modeling of organ failure, microvascular function in the clinic is mostly used to model human disease and has not fully implemented in diagnosis or therapy.

Fat tissue control of microvascular function
We have shown that fat tissue around blood vessels, or perivascular adipose tissue (PVAT) in regulation of normal and impaired organ blood flow. For instance, reduced muscle perfusion contributes to insulin resistance and reduced exercise capacity in obesity and type 2 diabetes, while microvascular inflammation and reduced perfusion impair myocardial function. This research started with our hypothesis paper published in the leading medical journal The Lancet and has resulted in evidence that PVAT controls muscle blood flow through a balance of vasodilator and vasoconstrictor products, known as adipokines. While the balance between these two is tilted towards vasodilator adipokines in normal physiology, it shifts towards vasoconstrictor adipokines in obesity, even in before diseases such as type 2 diabetes occur. We have also shown that kidney failure, a complication of obesity and diabetes, has a separate and aggravating effect on organ blood flow through reduction of the synthesis of nitric oxide.
Expertise in microvascular function and metabolism
As we study interactions between fat tissue function, organ blood flow and glucose metabolism, we have expertise in analysis and manipulation of these characteristics. Specific techniques used in our group:
- Vessel myography
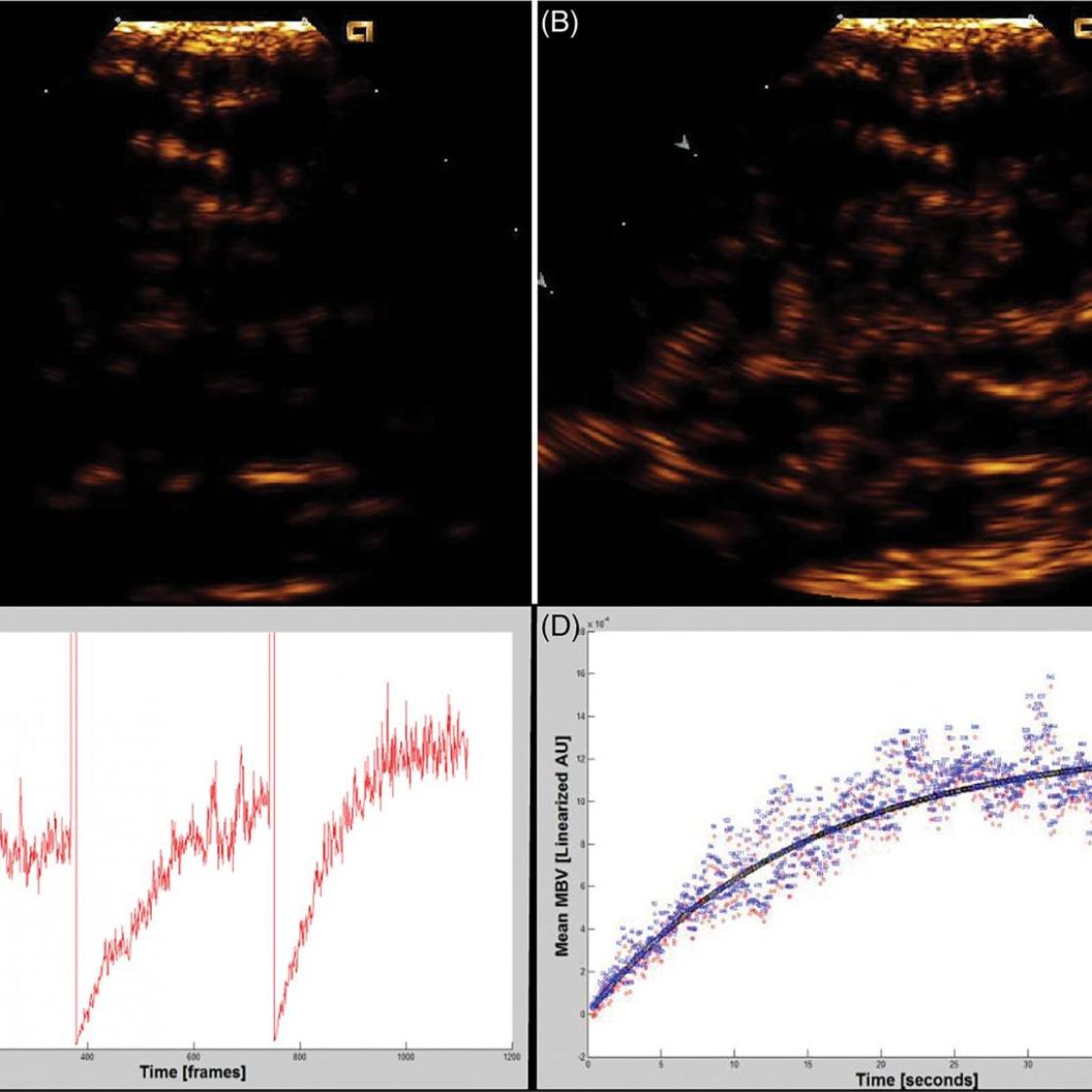
- Blood flow imaging with contrast ultrasonography
- Glucose metabolism: hyperinsulinemic, euglycemic clamp
- Adipose tissue phenotyping: protein, RNA, histology
- Manipulation of fat tissue in vivo
- Modeling of microvascular dysfunction in vivo and in vitro

Collaboration
National
- Amsterdam UMC: Erik Serné, Marc Vervloet, Jolanda van der Velden, Max Nieuwdorp
- Erasmus MC: Dirk Duncker
- LUMC: Patrick Rensen, Marie-Jose Goumans
International
- Anthony Heagerty, University of Manchester
- Michelle Keske, University of Melbourne
Current funding (external)
Netherlands Heart Foundation, consortium grant (CVON Reconnect)
Netherlands Heart Foundation, innovation grant
Netherlands Heart Institute, project grant
European Union, European Training Network grant (IMPROVE-PD)
Recent funding (2018-19)
Netherlands Organisation for Scientific Research (NWO), Vidi grant
Danish research foundation
Novo Nordisk
Netherlands Kidney Foundation (consortium grant)
Recent publications
- Padro T, Manfrini O, Bugiardini R, Canty J, Cenko E, De Luca G, Duncker DJ, Eringa EC, Koller A, Tousoulis D, Trifunovic D, Vavlukis M, de Wit C and Badimon L. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on 'coronary microvascular dysfunction in cardiovascular disease'. Cardiovasc Res. 2020.
- Emanuel AL, Meijer RI, van Poelgeest E, Spoor P, Serné EH and Eringa EC. Contrast-enhanced ultrasound for quantification of tissue perfusion in humans. Microcirculation. 2020;27:e12588.
- Turaihi AH, Serne EH, Molthoff CFM, Koning JJ, Knol J, Niessen HW, Goumans M, van Poelgeest EM, Yudkin JS, Smulders YM, Jimenez CR, van Hinsbergh VWM and Eringa EC. Perivascular Adipose Tissue Controls Insulin-Stimulated Perfusion, Mitochondrial Protein Expression, and Glucose Uptake in Muscle Through Adipomuscular Arterioles. Diabetes. 2020;69:603-613.
- Emanuel AL, van Duinkerken E, Wattjes MP, Klein M, Barkhof F, Snoek FJ, Diamant M, Eringa EC, IJzerman RG and Serné EH. The presence of cerebral white matter lesions and lower skin microvascular perfusion predicts lower cognitive performance in type 1 diabetes patients with retinopathy but not in healthy controls—A longitudinal study. Microcirculation. 2019;26:e12530.
- Saxton SN, Clark BJ, Withers SB, Eringa EC and Heagerty AM. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiological Reviews. 2019;99:1701-1763.
- Meijer RI, Hoevenaars FPM, Serne EH, Yudkin JS, Kokhuis TJA, Weijers EM, van Hinsbergh VWM, Smulders YM and Eringa EC. JNK2 in myeloid cells impairs insulin's vasodilator effects in muscle during early obesity development through perivascular adipose tissue dysfunction. Am J Physiol Heart Circ Physiol. 2019;317:H364-h374.
- Meekel J, Dias-Neto M, Bogunovic N, Lely R, Eringa E, Wisselink W, Blankensteijn J and Yeung K. Increased Intra-individual Perivascular Adipose Tissue Density and Increased Inflammatory RNA Expression of Perivascular Adipose Tissue in Patients with Abdominal Aortic Aneurysms. European Journal of Vascular and Endovascular Surgery. 2019;58:e349-e350.
- Turaihi AH, Bakker W, van Hinsbergh VWM, Serné EH, Smulders YM, Niessen HWM and Eringa EC. Insulin Receptor Substrate 2 Controls Insulin-Mediated Vasoreactivity and Perivascular Adipose Tissue Function in Muscle. Front Physiol. 2018;9:245.
- Verkaik M, Oranje M, Abdurrachim D, Goebel M, Gam Z, Prompers JJ, Helmes M, Ter Wee PM, van der Velden J, Kuster DW, Vervloet MG and Eringa EC. High Fibroblast Growth Factor 23 concentrations in experimental renal failure impair calcium handling in cardiomyocytes. Physiological reports. 2018;6:e13591.
- Verkaik M, Juni RP, van Loon EPM, van Poelgeest EM, Kwekkeboom RFJ, Gam Z, Richards WG, Ter Wee PM, Hoenderop JG, Eringa EC and Vervloet MG. FGF23 impairs peripheral microvascular function in renal failure. Am J Physiol Heart Circ Physiol. 2018;315:H1414-h1424.
- Emanuel AL, de Clercq NC, Koopen AM, van Poelgeest E, Serlie MJM, van Raalte DH, Kramer MHH, Nieuwdorp M, Eringa EC and Serné EH. Iloprost infusion prevents the insulin-induced reduction in skeletal muscle microvascular blood volume but does not enhance peripheral glucose uptake in type 2 diabetic patients. Diabetes Obes Metab. 2018;20:2523-2531.
- Turaihi AH, van Poelgeest EM, van Hinsbergh VW, Serne EH, Smulders YM and Eringa EC. Combined Intravital Microscopy and Contrast-enhanced Ultrasonography of the Mouse Hindlimb to Study Insulin-induced Vasodilation and Muscle Perfusion. J Vis Exp. 2017.