Endometriosis
Endometriosis is a multi-factorial disease in which genetic predisposition, epigenetics, steroid signalling and metabolism, immune response, angiogenesis and other factors play a role. We use distinct scientific and multi-disciplinary approaches to address several aspects in the pathogenic mechanism. Our final aim is to improve diagnosis and to improve treatments.
Endometriosis is characterised by the presence of endometrium outside the uterine cavity (ectopic location). Around 10 % of all women during pre-menopause suffer form endometriosis. Several distressing features accompany this disease, including pelvic pain, dysmenorrhoea and infertility. All these factors have an important impact on the social, professional and marital life of women. Endometriosis is a multi-factorial disorder and several events are responsible for the translocation of the endometrial tissue inside the peritoneum, its survival and attachment to the peritoneal cavity and other locations and for its growth. These include angiogenesis to supply endometriotic tissue with oxygen and nutrients, estrogen signalling to stimulate growth, diminished immune surveillance and epigenetic aberrations. In addition, endometriosis shows familiar clustering, indicating a probable genetic predisposition in the development of this disorder.
Angiogenesis, estrogen metabolism, genetic predisposition and epigenetics are all areas of research on which our group is focussing.
Estrogen metabolism
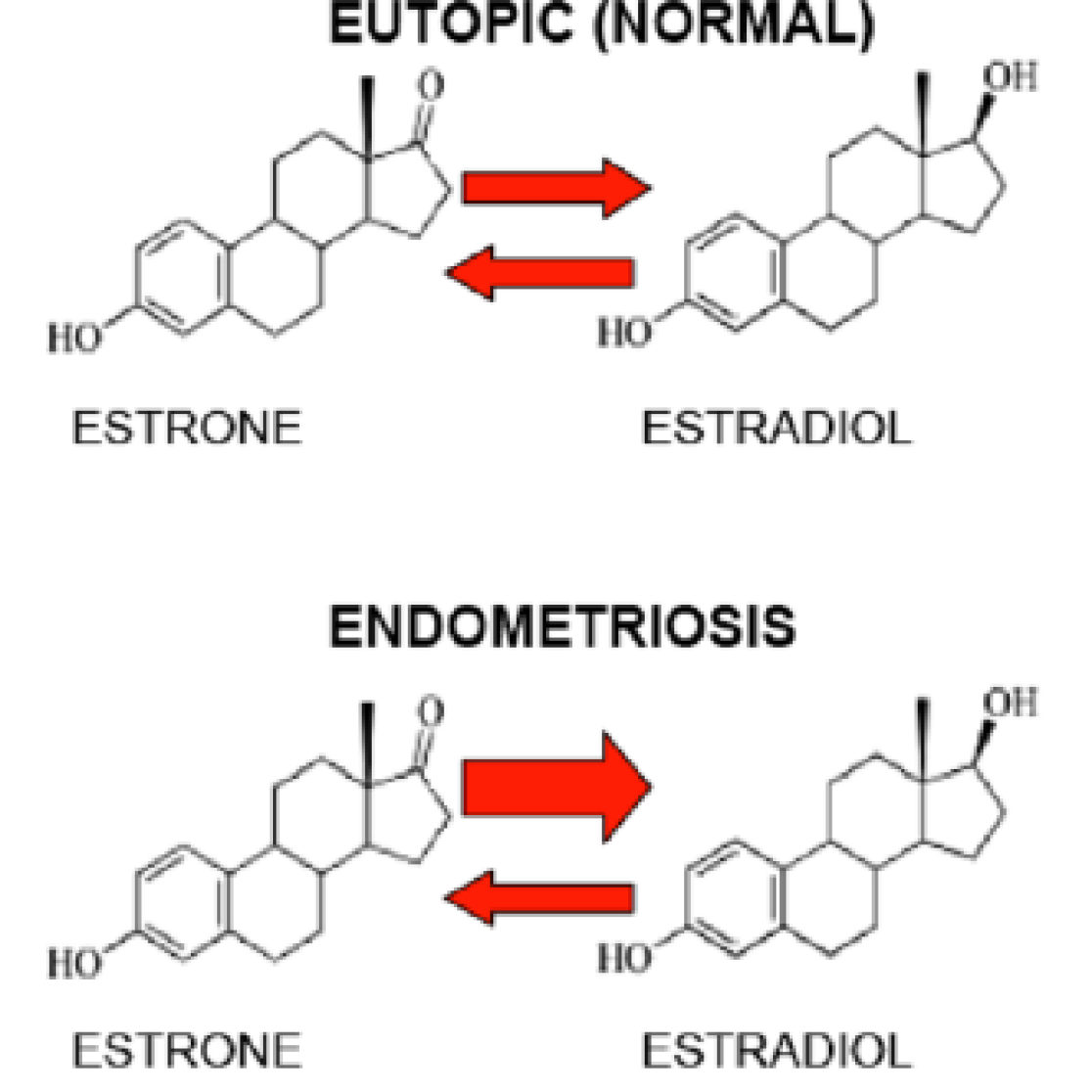
By using our HPLC-based method to measure the metabolism of estrogens in endometriosis patients, we have shown that the equilibrium in the activation/de-activation of 17β-estradiol in the ectopic location is shifted towards an increased synthesis and exposure to 17β-estradiol.
Such unbalanced estrogen metabolisms is therefore responsible for the synthesis of estrogens inside the endometriotic tissues, which, in turn, supports its own growth. Recently, in a collaborative effort with the drug discovery company Forendo Pharma, we have shown that newly developed compounds can inhibit this reaction. They represent promising future drugs for endometriosis and a pipeline to bring these compounds to the market is underway (see also Forendo Pharma press release).
Diagnosis
A non-invasive diagnostic test for endometriosis does not exist. Currently, the gold diagnostic standard is laparoscopy, which requires a surgical operation. Therefore, the diagnosis can be delayed of 8-11 years after first symptom presentation. Given the distressing features associated with this disease, a delayed diagnosis is detrimental for the life of all women suffering from endometriosis.
The high angiogenic activity of endometriosis can be exploited for diagnosis. Blood vessels and can be easily visualised by magnetic resonance imaging (MRI). Contrast agents can be used to improve the MRI contrast and the diagnostic power.
Using a mouse model of endometriosis, we have shown that this technique may be used to develop a non-invasive diagnostic test for endometriosis. As indicated in the image below, the signal intensity of the injected contrast agent (i.e. the detection power) decreases with time in the control tissue but remains high in endometriosis, making feasible its detection by MRI.
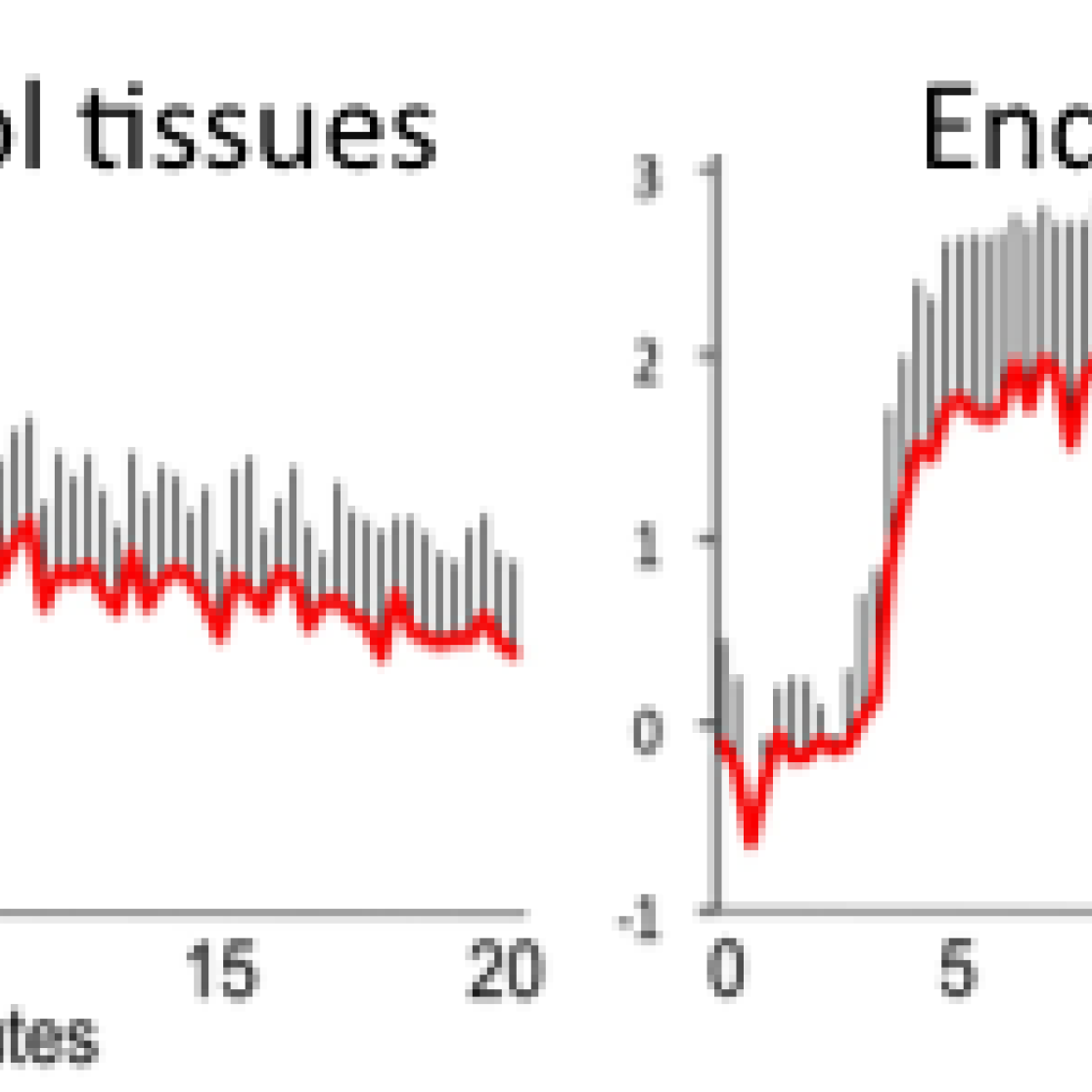
Diagnostic imaging
Based on these results in a small-animal model, a trial to test the feasibility of contrast enhanced MRI to diagnose endometriosis in humans has been initiated (Nederlands Trial Register NTR3738). This study is a collaboration with the Department of Radiology, where large expertise on diagnostic imaging in oncology has been gathered during the past few years.
Genetic predisposition
Polymorphisms or variants at genes involved in steroid signalling and immune response have been investigated for possible association with endometriosis risk. Case control studies have been performed to assess the influence on endometriosis of the following gene variants: PROGINS (Reference SNP Cluster Report: rs1042838) and +331G/A (rs10895068) polymorphisms in the progesterone receptor gene; –509C/T polymorphism (rs1800469) in the transforming Growth Factor β1 gene; –817C>T polymorphism (rs9514828) in the B lymphocyte stimulator gene.
Molecular mechanism of pathogenesis
Proteins, biological and cellular events that may be involved in the distinct steps of endometriosis initiation, establishment and maintenance are investigated, in search for potential therapeutic and diagnostic targets. In this context, we have identified an extracellular matrix protein, olfactomedin-4, which may be particularly important in the early events of endometriosis, i.e. the spread of endometrial cells and their survival in the peritoneal cavity. A second study has indicated that paired-box-2, a protein involved in the genital tract development during embryogenesis and it its maintenance during adultness, is highly expressed in endometrial cells and may be exploited as a diagnostic biomarker.
Angiogenesis
Angiogenesis (i.e. the formation of novel blood vessels) plays a key role in endometriosis. This process and the underlying molecular factors responsible for its regulation have been investigated by our research team. We have explored the possibility of inhibiting the formation of new vessels to treat endometriosis using experimental models. Several studies, including ours, suggest that the vasculature of endometriotic lesions may express a specific panel of markers. These characteristics may be exploited to develop endometriosis selective anti-angiogenic drugs in the future.
Economic and social impact of endometriosis
Although some women with endometriosis are asymptomatic, a substantial part of the patients suffer from severe dysmenorrhoea, chronic pelvic pain, deep dyspareunia, dysuria and infertility. These symptoms can cause severe discomfort and alter women’s quality of life. In this line of research we investigate some aspects of quality of life in endometriosis patients:
- Long term follow-up of quality of life after resection by laparotomy of deeply infiltrating endometriosis involving the bowel.
- The direct and indirect cost of endometriosis with a view to determine the overall socio-economic impact of endometriosis.
This research is embedded within an international multicentre study (EndoCost Study).
Selected publications
Delvoux et al. J Clin Endocrinol Metab. 2014 99(1):278-284.
Schreinemacher M et al. PlosOne 2012 7(3):e33241.
De Graaff A et al. Hum Reprod. 2012 27(6):1676-84.
Simoens S et al. Gynecol Obstet Invest. 2011 71:170-6.
De Graaff A et al. Fertil Steril. 2010 94(3):1108-10.
Dassen H et al. Am J Pathol. 2010 177(5)_2495-508.
Delvoux B et al. J Clin Endocrinol Metab. 2009 94(3):876-83.
van Kaam KJ et al. Hum Reprod. 2008 23(12):2692-700.
van Kaam KJ et al. Reprod Sci. 2007 May;14(4):367-73.
Romano A et al. J Mol Endocrinol. 2007 38(1-2):331-50.
Van Langendonckt A et al. Mol Hum Reprod. 2007 13(12):875-86.
van Kaam KJ et al. Hum Reprod. 2007 22(1):129-35.
Nap A et al. J Clin Endocrinol Metab. 2004 89(3):1089-95.