Protein accumulation mapped for Alzheimer’s
Researchers at Maastricht University – together with colleagues from German research institutes – have managed for the first time to map exactly how the amyloid beta protein accumulates in the brains of people with Alzheimer's disease. According to the researchers, amyloid beta plays an important role in the disease together with the tau protein. The M4I Division of Nanoscopy was able to chart the molecular structure thanks to the huge cryo-electron microscope in Maastricht. The results and images were published in the leading academic journal Science on 7 September.
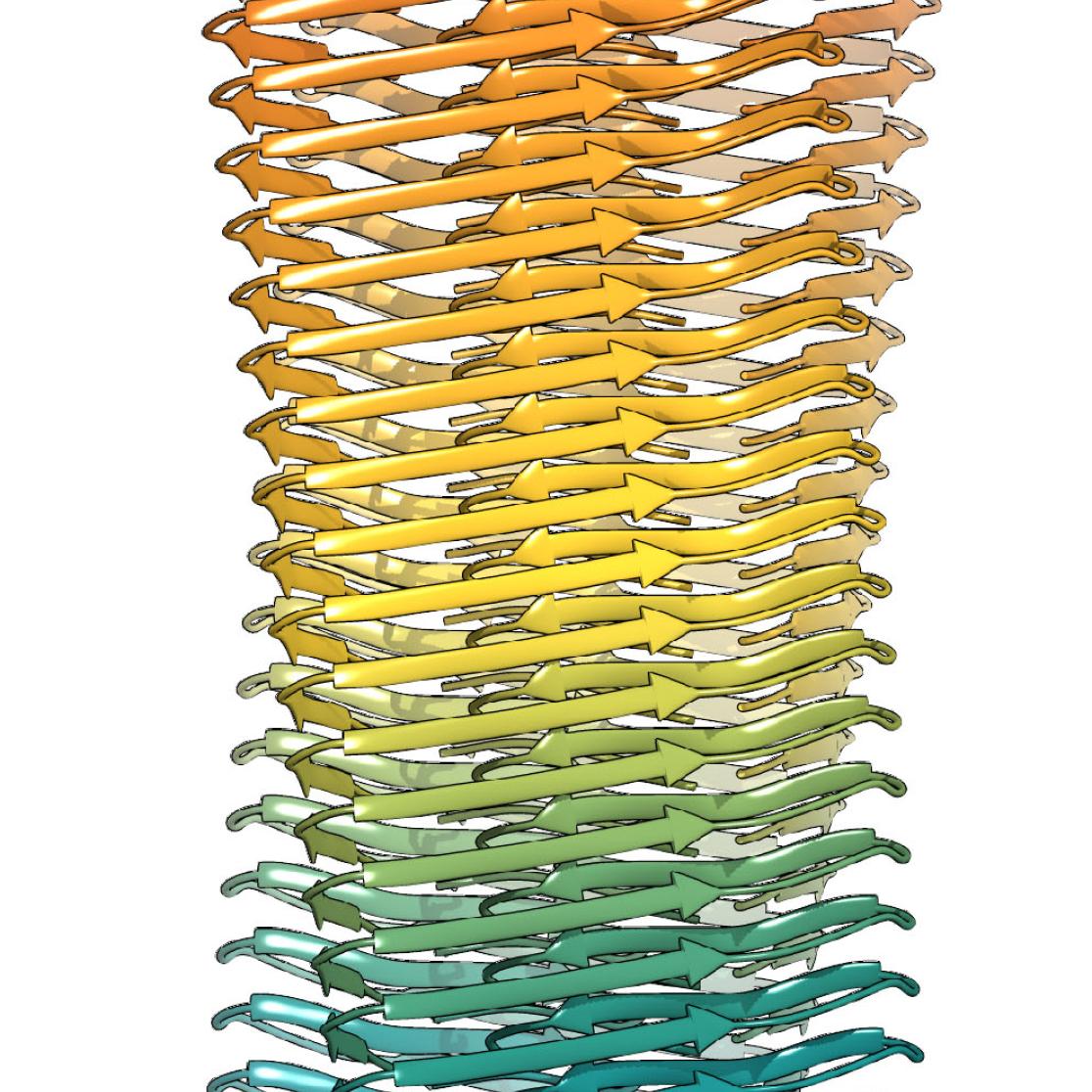
Amyloid beta is found in large amounts within all human brains. Whereas excess proteins are broken down in healthy people, however, they accumulate in the brains of people with Alzheimer's disease. This accumulation of amyloid beta has now been mapped by Carmen Lopez Iglesias and Raimond Ravelli from Maastricht, together with colleagues from Jülich, Düsseldorf and Hamburg. The cryo-electron microscope in the lab managed by Professor Peter Peters in Maastricht plays a crucial part in this process.
The molecular structure of another crucial 'Alzheimer's protein', tau, was published in Nature last month by the scientist Sjors Scheres, who was born in Limburg and is a professor at the MRC Laboratory for Molecular Biology in Cambridge.
The protein model
In the Science article, the researchers use the molecular structure to describe how specific known gene mutations can either promote or prevent these protein accumulations in Alzheimer's disease. Researcher Raimond Ravelli: 'The structure provides insight into the clustering of this protein and the amino acids which play a special part here.' Greater insight into such processes can help scientists to develop drugs which dismantle these protein accumulations, for example. You can watch a short animation here visualising raw recordings of beta-amyloid fibrils with the 3D atomic structure superimposed.
Cryo-electron microscope
The Maastricht cryo-electron microscope can examine cell structures at a temperature of -185°C, making it possible to study cell structures without having to process them with toxic chemicals. Cells are frozen extremely quickly, after which they can be studied in more detail than ever before. Peter Peters has previously described this development as follows: 'The switch in technology – from the conventional electron microscope to cryo-electron tomography – could be compared to the transformation from the old X-rays to the new CT scan.'
The Science publication is entitled: ‘Fibril structure of amyloid-β (1-42) by cryoelectron microscopy’. The title of the Nature publication is: ‘Cryo-EM structures of tau filaments from Alzheimer’s disease’.
Also read
-
Global Citizenship for Sustainable Development grant winners announced
We are proud to announce that three projects have received funding from the Global Citizenship for Sustainable Development network at Maastricht University.

-
Inaugural lecture Jan Willem van Prooijen
What drives people to embrace radical conspiracy theories, sometimes with far-reaching consequences for society? During his inaugural lecture on Friday 27 June, Prof. Dr. Jan Willem van Prooijen (radicalisation, extremism, and conspiracy thinking) will address this urgent question.

-
Update 25 June
Last night a UM building at the Bouillonstraat was daubed with paint and slogans. A sad expression of vandalism. UM has filed a report.
