Coll-a-Gen
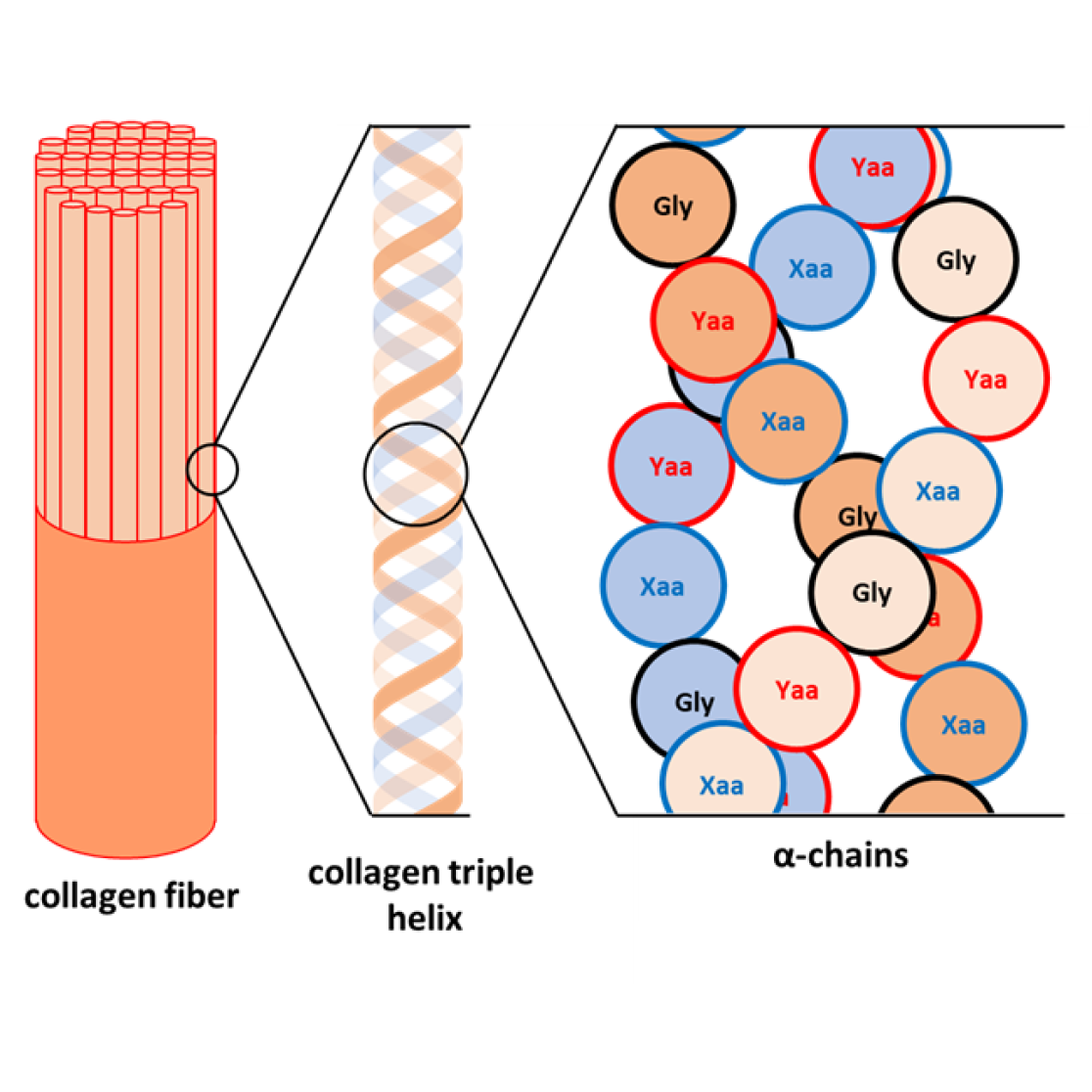
Collagen, a protein essential for the structural integrity of various biological tissues such as bones, skin, and cartilage, owes its exceptional mechanical properties to a unique triple helix structure composed of three intertwined polypeptide chains.
This structure, rich in proline and hydroxyproline, enables the formation of a stable triple-helical structure through hydrogen bonding. The unique structure and properties of collagen have led to an increasing demand for its applications across fields, ranging from tissue engineering and wound healing to bioplastics and coatings. Currently, collagen is primarily sourced from animals, a process hampered by challenges including complex extraction methods, the risk of disease transmission, and variability in yield and quality. Moreover, alternative production methods via synthetic biology face hurdles such as low yields, high costs, and the complexity associated with replicating post-translational modifications. While chemical synthesis represents a promising alternative, it too is constrained by high costs, labour intensity, and limitations on the length of peptide chains, which generally do not exceed 60 amino acids.
Recognizing these challenges, there is a clear and pressing demand for a scalable and sustainable source of collagen. Despite the mentioned limitations of chemical synthesis, primarily through solid-phase peptide synthesis (SPPS), it has shown promise in synthesizing short model systems, known as collagen mimic peptides (CMPs), which replicate specific structural or functional attributes of natural collagen. In this interdisciplinary project, we aim to explore new synthetic approaches for CMPs that could help bridge the gap between synthetic and natural collagen.