Movement
Crossroad
Research theme: Neuroimaging
Clinical pillar: Movement
A better understanding of the anatomical-functional organisation of the human brain is crucial for precision medicine and development of future disease-modifying therapies in movement disorders such as Parkinson’s disease and related disorders. Low field as well as ultra-high field magnetic resonance imaging is used as a powerful tool to diagnose and monitor the principles of the pathophysiology of movement disorders, with a focus on both motor and non-motor (neuropsychiatric, cognitive) symptoms, and fine-tune clinical applications such as deep brain stimulation.
Unique contributions and highlights
As disease-modifying therapies for movement disorders are being developed, early detection and reliable monitoring of clinical symptoms and disease progression is pivotal. We determined correlates of the complex neural network involved in non-motor symptoms of PD such as emotional processing (Moonen et al. PLoS One. 2017, Carey et al. Mov. Dis. 2021), apathy (Reijnders et al. Mov Disord. 2010) and cognitive impairment (Wolters et al. Parkinsonism Relat Disord. 2019).
A grant from the Weijerhorst foundation was awarded to take the next step in the Understand-Track-Adjust Parkinson’s disease (UTAP) project. Ultra-high field imaging at 7T is now applied in early PD patients diagnosed within 3 years of diagnosis and monitored for 4 years in an unique large cohort of 105 PD patients and compared with healthy controls within the TRACK-PD study, in collaboration with the imaging group at the Faculty of Psychology and Neuroscience and Scannexus. The baseline cohort is now complete and analysis of imaging correlates with a detailed set of genetic, motor and non-motor symptoms is now taking place (Wolters et al. BMC Neurol. 2020). The follow-up assessments and visits are still running. This study is coupled to a post-mortem study of 20 brains of PD patients that will be investigated with 9.4T MRI. The first progress made in this approach is the development of a custom-built nonferromagnetic container that allows whole brain hemispheres to be scanned at sub-millimeter resolution (Boonstra et al. Neuroimage 2021).
Ultra-high-field imaging holds promise to become the new gold standard for stereotaxic neurosurgery (Isaacs et al. Trends Biotechnol. 2017). In order to inform treatment planning directly, the organization of brain targets for deep brain stimulation (DBS) was studied in 17 PD patients scheduled for DBS to fine-tune the placement of electrodes and minimize side effects of stimulation. In collaboration with University of Minnesota (USA) in Mineapolis, we performed a segmentation of the nucleus subthalamicus (STN) as a target for DBS (Plantinga et al. Neuroimage. 2018) and found there is a considerable variation of the motor, limbic and associative areas within the STN. In addition, we compared DBS target coordinates planned by different neurosurgeons at different MRI field strengths (3T versus 7T) and found that the actual selected location of the electrode is seemingly more ventral when the 3T scan was used (Isaacs et al. Neuroimage 2021). The use of ultra-high-field imaging in stereotaxic neurosurgery and planning is now further tested in a larger cohort of patients.
Another opportunity for clinical translational arises from the development of fMRI-based neurofeedback protocols for Parkinson’s disease (Subramanian et al., J Neurosci. 2011). Within the NEURIPIDES consortium this is now further deployed and includes simultaneous DBS-fMRI studies and anatomical connectivity mapping of the cortico-subcortical networks supporting symptom relief, classification analysis of therapeutic activation patterns, and proof of concept of the feasibility of neurofeedback with these signals.
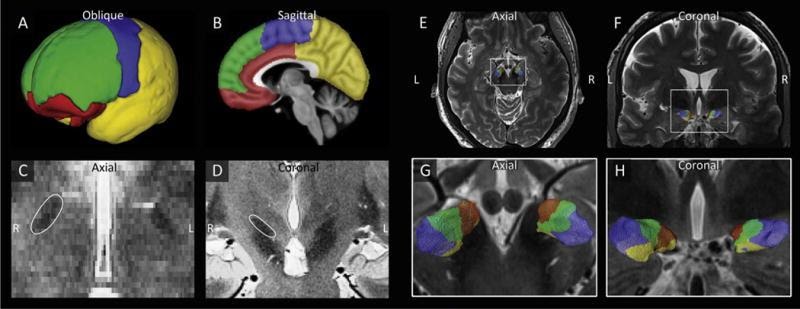
7T-image of STN-parcellation in a Parkinson’s disease patient. Plantinga et al. Neuroimage 2018.
